- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Bexarotene Retinoid Receptor agonist
Cat.No.S2098
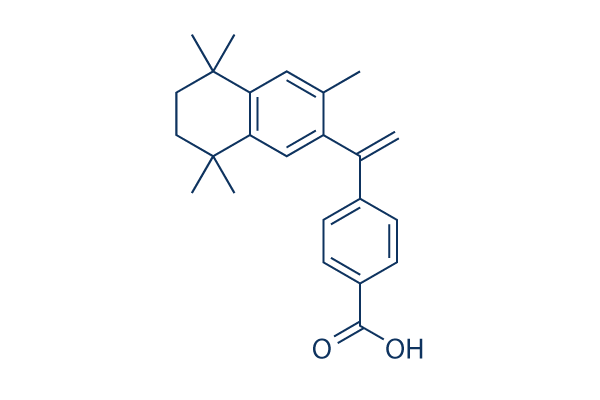
Chemical Structure
Molecular Weight: 348
Jump to
Quality Control
Batch:
Purity:
99.89%
99.89
| Related Targets | Dehydrogenase HSP Transferase P450 (e.g. CYP17) PDE phosphatase PPAR Vitamin Carbohydrate Metabolism Mitochondrial Metabolism |
|---|---|
| Other Retinoid Receptor Inhibitors | TTNPB AM580 Tamibarotene UVI 3003 Fenretinide SR 11237 BMS493 Palovarotene CD1530 AR7 |
Solubility
|
In vitro |
DMSO
: 70 mg/mL
(201.14 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 348 | Formula | C24H28O2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 153559-49-0 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | LGD1069 | Smiles | CC1=CC2=C(C=C1C(=C)C3=CC=C(C=C3)C(=O)O)C(CCC2(C)C)(C)C | ||
Mechanism of Action
| Targets/IC50/Ki |
RXR
|
|---|---|
| In vitro |
Bexarotene treatment at 1 mM and 10 mM for 96 h increases the number of cells with sub-G1 populations and annexin V binding in a dose-dependent manner compared with vehicle controls (DMSO) in well-established CTCL cell lines (MJ, Hut78, and HH), respectively. This compound suppresses the expression of retinoid X receptor alpha and retinoic acid receptor alpha proteins in all three lines compared with untreated controls. It decreases the protein levels of survivin, activates caspase-3, and cleaved poly(ADP-Ribose) polymerase, but has no obvious effect on expression of Fas/Fas ligand and bcl-2 proteins in all three CTCL lines. It induces a loss of viability and more pronounced inhibition of clonogenic proliferation in HH and Hut-78 cells, whereas the MJ line exhibits resistance. The chemical upregulates and activates Bax in sensitive lines, although not enough to signal significant apoptosis. It signals both G(1) and G(2)/M arrest by the modulation of critical checkpoint proteins. It activates p53 by phosphorylation at Ser15, which influences the binding of p53 to promoters for cell cycle arrest, induces p73 upregulation, and, in concordance, also modulates some p53/p73 downstream target genes, such as p21, Bax, survivin and cdc2.
|
| In vivo |
Bexarotene significantly prevents ER-negative mammary tumorigenesis with less toxicity than naturally occurring retinoids in animal models. This compound inhibits the development of preinvasive mammary lesions such as hyperplasias and carcinoma-in-situ in MMTV-erbB2 mice.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT03323658 | Completed | Breast Atypical Ductal Hyperplasia|Breast Atypical Lobular Hyperplasia|Breast Ductal Carcinoma In Situ|Breast Lobular Carcinoma In Situ|Invasive Breast Carcinoma |
National Cancer Institute (NCI) |
June 15 2018 | Phase 1 |
| NCT01569724 | Completed | Hypertriglyceridemia|Cutaneous T Cell Lymphoma |
Rennes University Hospital |
January 2012 | Phase 4 |
| NCT01001143 | Completed | Leukemia Myeloid Acute |
Washington University School of Medicine |
May 2010 | Phase 1 |
| NCT00615784 | Terminated | Acute Myeloid Leukemia |
Abramson Cancer Center at Penn Medicine |
May 25 2010 | Phase 2 |
| NCT00125372 | Completed | Carcinoma Non-small-cell Lung |
Dartmouth-Hitchcock Medical Center|Ligand Pharmaceuticals|Genentech Inc. |
December 2005 | Not Applicable |
| NCT00316030 | Completed | AML|Acute Myeloid Leukemia |
Abramson Cancer Center at Penn Medicine |
January 2004 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































