
- Bioactive Compounds
- By Signaling Pathways
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- DNA Damage/DNA Repair
- Compound Libraries
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-Ⅱ
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Plant Extract Library
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at info@selleckchem.com to customize your library.
You could select:
- Antibodies
- Bioreagents
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- Protein Assay
- Protein A/G Magnetic Beads for IP
- Anti-Flag magnetic beads
- Anti-Flag Affinity Gel
- Anti-Myc magnetic beads
- Anti-HA magnetic beads
- Poly DYKDDDDK Tag Peptide lyophilized powder
- Protease Inhibitor Cocktail
- Protease Inhibitor Cocktail (EDTA-Free, 100X in DMSO)
- Phosphatase Inhibitor Cocktail (2 Tubes, 100X)
- Cell Biology
- Cell Counting Kit-8 (CCK-8)
- Animal Experiment
- Mouse Direct PCR Kit (For Genotyping)
- New Products
- Contact Us
Tipifarnib
FTase inhibitor
research use only
Tipifarnib is a potent and specific farnesyltransferase (FTase) inhibitor with IC50 of 0.6 nM, its anti-proliferative effects are most prominent in H-ras or N-ras mutant cells. Phase 3.
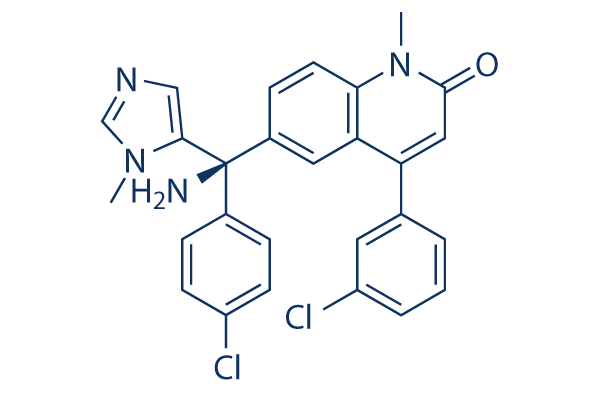
Tipifarnib Chemical Structure
Molecular Weight: 489.4
Purity & Quality Control
Batch:
Purity:
99.88%
99.88
Tipifarnib Related Products
| Related Products | FTI 277 HCl | Click to Expand |
|---|---|---|
| Related Compound Libraries | Metabolism Compound Library Anti-cancer Metabolism Compound Library Glutamine Metabolism Compound Library Carbohydrate Metabolism Compound Library Lipid Metabolism Compound Library | Click to Expand |
Cell Culture and Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| Sf9 cells | Function assay | Inhibition of rat recombinant PFT expressed in insect Sf9 cells by scintillation proximity assay, IC50=0.7 nM | 20429511 | |||
| NIH3T3 cells | Function assay | Inhibition of Ras processing in H-ras transformed NIH3T3 cells in presence of Tipifarnib, EC50=1.6 nM | 15911281 | |||
| NIH 3T3 | Function assay | Effective concentration against Ha-RAS processing in NIH3T3 ras-transformed cells, EC50 = 0.002 μM. | 12657284 | |||
| NIH 3T3 | Function assay | Inhibition of T24F1H-ras-transformation in NIH 3T3 cells, IC50 = 0.0017 μM. | 12699751 | |||
| NIH 3T3 | Function assay | Inhibition of Ras farnesylation in H-Ras transformed NIH3T3 cells, EC50 = 0.0016 μM. | 15454228 | |||
| Sf9 | Function assay | Inhibition of rat recombinant protein farnesyltransferase expressed in Sf9 cells by [3H]-scintillation proximity assay, IC50 = 0.0007 μM. | 19239254 | |||
| 3T3 | Antitrypanosomal assay | Antitrypanosomal activity against Trypanosoma cruzi Tulahuen infected in mouse 3T3 cells, EC50 = 0.004 μM. | 19875282 | |||
| 3T3 | Antitrypanosomal assay | 7 days | Antitrypanosomal activity against Trypanosoma cruzi Tulahuen amastigotes infected in rat 3T3 cells after 7 days by alamar blue assay, EC50 = 0.004 μM. | 20429511 | ||
| osteosarcoma cells | Function assay | Inhibition of FTase isolated from Kirsten virus-transformed human osteosarcoma cells using K-rasB peptide as substrate in presence of [3H]farnesyl PPi by scintillation proximity assay, IC50 = 0.0079 μM. | 28505447 | |||
| RPMI-6666 | Growth inhibition assay | Inhibition of human RPMI-6666 cell growth in a cell viability assay, IC50 = 0.007407 μM. | SANGER | |||
| ML-2 | Growth inhibition assay | Inhibition of human ML-2 cell growth in a cell viability assay, IC50 = 0.01364 μM. | SANGER | |||
| SIG-M5 | Growth inhibition assay | Inhibition of human SIG-M5 cell growth in a cell viability assay, IC50 = 0.01457 μM. | SANGER | |||
| QIMR-WIL | Growth inhibition assay | Inhibition of human QIMR-WIL cell growth in a cell viability assay, IC50 = 0.01631 μM. | SANGER | |||
| A4-Fuk | Growth inhibition assay | Inhibition of human A4-Fuk cell growth in a cell viability assay, IC50 = 0.02059 μM. | SANGER | |||
| ETK-1 | Growth inhibition assay | Inhibition of human ETK-1 cell growth in a cell viability assay, IC50 = 0.02152 μM. | SANGER | |||
| MEL-JUSO | Growth inhibition assay | Inhibition of human MEL-JUSO cell growth in a cell viability assay, IC50 = 0.02344 μM. | SANGER | |||
| NALM-6 | Growth inhibition assay | Inhibition of human NALM-6 cell growth in a cell viability assay, IC50 = 0.03621 μM. | SANGER | |||
| IA-LM | Growth inhibition assay | Inhibition of human IA-LM cell growth in a cell viability assay, IC50 = 0.04752 μM. | SANGER | |||
| Daoy | Growth inhibition assay | Inhibition of human Daoy cell growth in a cell viability assay, IC50 = 0.05753 μM. | SANGER | |||
| REH | Growth inhibition assay | Inhibition of human REH cell growth in a cell viability assay, IC50 = 0.0592 μM. | SANGER | |||
| KU812 | Growth inhibition assay | Inhibition of human KU812 cell growth in a cell viability assay, IC50 = 0.09706 μM. | SANGER | |||
| KM12 | Growth inhibition assay | Inhibition of human KM12 cell growth in a cell viability assay, IC50 = 0.11686 μM. | SANGER | |||
| LCLC-103H | Growth inhibition assay | Inhibition of human LCLC-103H cell growth in a cell viability assay, IC50 = 0.12902 μM. | SANGER | |||
| BC-1 | Growth inhibition assay | Inhibition of human BC-1 cell growth in a cell viability assay, IC50 = 0.12996 μM. | SANGER | |||
| CMK | Growth inhibition assay | Inhibition of human CMK cell growth in a cell viability assay, IC50 = 0.15145 μM. | SANGER | |||
| MCF7 | Growth inhibition assay | Inhibition of human MCF7 cell growth in a cell viability assay, IC50 = 0.1663 μM. | SANGER | |||
| Calu-1 | Growth inhibition assay | Inhibition of human Calu-1 cell growth in a cell viability assay, IC50 = 0.18134 μM. | SANGER | |||
| SK-LMS-1 | Growth inhibition assay | Inhibition of human SK-LMS-1 cell growth in a cell viability assay, IC50 = 0.18409 μM. | SANGER | |||
| HH | Growth inhibition assay | Inhibition of human HH cell growth in a cell viability assay, IC50 = 0.19089 μM. | SANGER | |||
| NCI-H2122 | Growth inhibition assay | Inhibition of human NCI-H2122 cell growth in a cell viability assay, IC50 = 0.19145 μM. | SANGER | |||
| SNG-M | Growth inhibition assay | Inhibition of human SNG-M cell growth in a cell viability assay, IC50 = 0.20354 μM. | SANGER | |||
| IGROV-1 | Growth inhibition assay | Inhibition of human IGROV-1 cell growth in a cell viability assay, IC50 = 0.20823 μM. | SANGER | |||
| HMV-II | Growth inhibition assay | Inhibition of human HMV-II cell growth in a cell viability assay, IC50 = 0.21578 μM. | SANGER | |||
| KYSE-270 | Growth inhibition assay | Inhibition of human KYSE-270 cell growth in a cell viability assay, IC50 = 0.21669 μM. | SANGER | |||
| NTERA-S-cl-D1 | Growth inhibition assay | Inhibition of human NTERA-S-cl-D1 cell growth in a cell viability assay, IC50 = 0.23744 μM. | SANGER | |||
| LoVo | Growth inhibition assay | Inhibition of human LoVo cell growth in a cell viability assay, IC50 = 0.24091 μM. | SANGER | |||
| MOLT-16 | Growth inhibition assay | Inhibition of human MOLT-16 cell growth in a cell viability assay, IC50 = 0.25823 μM. | SANGER | |||
| P30-OHK | Growth inhibition assay | Inhibition of human P30-OHK cell growth in a cell viability assay, IC50 = 0.26388 μM. | SANGER | |||
| HUTU-80 | Growth inhibition assay | Inhibition of human HUTU-80 cell growth in a cell viability assay, IC50 = 0.28502 μM. | SANGER | |||
| MIA-PaCa-2 | Growth inhibition assay | Inhibition of human MIA-PaCa-2 cell growth in a cell viability assay, IC50 = 0.3204 μM. | SANGER | |||
| HCC2157 | Growth inhibition assay | Inhibition of human HCC2157 cell growth in a cell viability assay, IC50 = 0.32316 μM. | SANGER | |||
| HCT-15 | Growth inhibition assay | Inhibition of human HCT-15 cell growth in a cell viability assay, IC50 = 0.32705 μM. | SANGER | |||
| 786-0 | Growth inhibition assay | Inhibition of human 786-0 cell growth in a cell viability assay, IC50 = 0.34156 μM. | SANGER | |||
| GDM-1 | Growth inhibition assay | Inhibition of human GDM-1 cell growth in a cell viability assay, IC50 = 0.35239 μM. | SANGER | |||
| NCI-H2009 | Growth inhibition assay | Inhibition of human NCI-H2009 cell growth in a cell viability assay, IC50 = 0.36336 μM. | SANGER | |||
| TE-15 | Growth inhibition assay | Inhibition of human TE-15 cell growth in a cell viability assay, IC50 = 0.36462 μM. | SANGER | |||
| NCI-H2342 | Growth inhibition assay | Inhibition of human NCI-H2342 cell growth in a cell viability assay, IC50 = 0.37983 μM. | SANGER | |||
| RT-112 | Growth inhibition assay | Inhibition of human RT-112 cell growth in a cell viability assay, IC50 = 0.38176 μM. | SANGER | |||
| HCC2998 | Growth inhibition assay | Inhibition of human HCC2998 cell growth in a cell viability assay, IC50 = 0.38436 μM. | SANGER | |||
| HEL | Growth inhibition assay | Inhibition of human HEL cell growth in a cell viability assay, IC50 = 0.39449 μM. | SANGER | |||
| NMC-G1 | Growth inhibition assay | Inhibition of human NMC-G1 cell growth in a cell viability assay, IC50 = 0.42505 μM. | SANGER | |||
| 8505C | Growth inhibition assay | Inhibition of human 8505C cell growth in a cell viability assay, IC50 = 0.43109 μM. | SANGER | |||
| HLE | Growth inhibition assay | Inhibition of human HLE cell growth in a cell viability assay, IC50 = 0.44811 μM. | SANGER | |||
| KGN | Growth inhibition assay | Inhibition of human KGN cell growth in a cell viability assay, IC50 = 0.45249 μM. | SANGER | |||
| EW-18 | Growth inhibition assay | Inhibition of human EW-18 cell growth in a cell viability assay, IC50 = 0.45791 μM. | SANGER | |||
| OCUB-M | Growth inhibition assay | Inhibition of human OCUB-M cell growth in a cell viability assay, IC50 = 0.47164 μM. | SANGER | |||
| SW620 | Growth inhibition assay | Inhibition of human SW620 cell growth in a cell viability assay, IC50 = 0.48224 μM. | SANGER | |||
| SK-MEL-2 | Growth inhibition assay | Inhibition of human SK-MEL-2 cell growth in a cell viability assay, IC50 = 0.48373 μM. | SANGER | |||
| G-401 | Growth inhibition assay | Inhibition of human G-401 cell growth in a cell viability assay, IC50 = 0.50261 μM. | SANGER | |||
| YKG-1 | Growth inhibition assay | Inhibition of human YKG-1 cell growth in a cell viability assay, IC50 = 0.5262 μM. | SANGER | |||
| ES6 | Growth inhibition assay | Inhibition of human ES6 cell growth in a cell viability assay, IC50 = 0.53935 μM. | SANGER | |||
| T84 | Growth inhibition assay | Inhibition of human T84 cell growth in a cell viability assay, IC50 = 0.5462 μM. | SANGER | |||
| HT-29 | Growth inhibition assay | Inhibition of human HT-29 cell growth in a cell viability assay, IC50 = 0.5467 μM. | SANGER | |||
| ALL-PO | Growth inhibition assay | Inhibition of human ALL-PO cell growth in a cell viability assay, IC50 = 0.55456 μM. | SANGER | |||
| A427 | Growth inhibition assay | Inhibition of human A427 cell growth in a cell viability assay, IC50 = 0.57917 μM. | SANGER | |||
| BB30-HNC | Growth inhibition assay | Inhibition of human BB30-HNC cell growth in a cell viability assay, IC50 = 0.58523 μM. | SANGER | |||
| A375 | Growth inhibition assay | Inhibition of human A375 cell growth in a cell viability assay, IC50 = 0.61648 μM. | SANGER | |||
| SNU-449 | Growth inhibition assay | Inhibition of human SNU-449 cell growth in a cell viability assay, IC50 = 0.62294 μM. | SANGER | |||
| A431 | Growth inhibition assay | Inhibition of human A431 cell growth in a cell viability assay, IC50 = 0.65536 μM. | SANGER | |||
| NCI-H1299 | Growth inhibition assay | Inhibition of human NCI-H1299 cell growth in a cell viability assay, IC50 = 0.69308 μM. | SANGER | |||
| SNU-423 | Growth inhibition assay | Inhibition of human SNU-423 cell growth in a cell viability assay, IC50 = 0.70466 μM. | SANGER | |||
| EFO-27 | Growth inhibition assay | Inhibition of human EFO-27 cell growth in a cell viability assay, IC50 = 0.71373 μM. | SANGER | |||
| SK-HEP-1 | Growth inhibition assay | Inhibition of human SK-HEP-1 cell growth in a cell viability assay, IC50 = 0.71407 μM. | SANGER | |||
| DMS-273 | Growth inhibition assay | Inhibition of human DMS-273 cell growth in a cell viability assay, IC50 = 0.71989 μM. | SANGER | |||
| MZ7-mel | Growth inhibition assay | Inhibition of human MZ7-mel cell growth in a cell viability assay, IC50 = 0.722 μM. | SANGER | |||
| SW1710 | Growth inhibition assay | Inhibition of human SW1710 cell growth in a cell viability assay, IC50 = 0.7255 μM. | SANGER | |||
| KYSE-450 | Growth inhibition assay | Inhibition of human KYSE-450 cell growth in a cell viability assay, IC50 = 0.72885 μM. | SANGER | |||
| MV-4-11 | Growth inhibition assay | Inhibition of human MV-4-11 cell growth in a cell viability assay, IC50 = 0.73279 μM. | SANGER | |||
| CAL-12T | Growth inhibition assay | Inhibition of human CAL-12T cell growth in a cell viability assay, IC50 = 0.76004 μM. | SANGER | |||
| KS-1 | Growth inhibition assay | Inhibition of human KS-1 cell growth in a cell viability assay, IC50 = 0.77035 μM. | SANGER | |||
| NCI-H650 | Growth inhibition assay | Inhibition of human NCI-H650 cell growth in a cell viability assay, IC50 = 0.77309 μM. | SANGER | |||
| NKM-1 | Growth inhibition assay | Inhibition of human NKM-1 cell growth in a cell viability assay, IC50 = 0.77418 μM. | SANGER | |||
| SUP-T1 | Growth inhibition assay | Inhibition of human SUP-T1 cell growth in a cell viability assay, IC50 = 0.78679 μM. | SANGER | |||
| CHL-1 | Growth inhibition assay | Inhibition of human CHL-1 cell growth in a cell viability assay, IC50 = 0.80546 μM. | SANGER | |||
| TE-10 | Growth inhibition assay | Inhibition of human TE-10 cell growth in a cell viability assay, IC50 = 0.81179 μM. | SANGER | |||
| MFH-ino | Growth inhibition assay | Inhibition of human MFH-ino cell growth in a cell viability assay, IC50 = 0.8145 μM. | SANGER | |||
| A388 | Growth inhibition assay | Inhibition of human A388 cell growth in a cell viability assay, IC50 = 0.86457 μM. | SANGER | |||
| TUR | Growth inhibition assay | Inhibition of human TUR cell growth in a cell viability assay, IC50 = 0.87119 μM. | SANGER | |||
| NCI-H727 | Growth inhibition assay | Inhibition of human NCI-H727 cell growth in a cell viability assay, IC50 = 0.87426 μM. | SANGER | |||
| CAL-148 | Growth inhibition assay | Inhibition of human CAL-148 cell growth in a cell viability assay, IC50 = 0.8842 μM. | SANGER | |||
| A3-KAW | Growth inhibition assay | Inhibition of human A3-KAW cell growth in a cell viability assay, IC50 = 0.90122 μM. | SANGER | |||
| TE-8 | Growth inhibition assay | Inhibition of human TE-8 cell growth in a cell viability assay, IC50 = 0.92847 μM. | SANGER | |||
| OAW-42 | Growth inhibition assay | Inhibition of human OAW-42 cell growth in a cell viability assay, IC50 = 0.9559 μM. | SANGER | |||
| CAL-39 | Growth inhibition assay | Inhibition of human CAL-39 cell growth in a cell viability assay, IC50 = 0.96232 μM. | SANGER | |||
| NCI-H1355 | Growth inhibition assay | Inhibition of human NCI-H1355 cell growth in a cell viability assay, IC50 = 0.97377 μM. | SANGER | |||
| 8305C | Growth inhibition assay | Inhibition of human 8305C cell growth in a cell viability assay, IC50 = 0.97631 μM. | SANGER | |||
| KYSE-510 | Growth inhibition assay | Inhibition of human KYSE-510 cell growth in a cell viability assay, IC50 = 0.9771 μM. | SANGER | |||
| COLO-679 | Growth inhibition assay | Inhibition of human COLO-679 cell growth in a cell viability assay, IC50 = 0.98506 μM. | SANGER | |||
| AN3-CA | Growth inhibition assay | Inhibition of human AN3-CA cell growth in a cell viability assay, IC50 = 1.00383 μM. | SANGER | |||
| DEL | Growth inhibition assay | Inhibition of human DEL cell growth in a cell viability assay, IC50 = 1.0259 μM. | SANGER | |||
| 5637 | Growth inhibition assay | Inhibition of human 5637 cell growth in a cell viability assay, IC50 = 1.02922 μM. | SANGER | |||
| BHY | Growth inhibition assay | Inhibition of human BHY cell growth in a cell viability assay, IC50 = 1.04579 μM. | SANGER | |||
| HCC70 | Growth inhibition assay | Inhibition of human HCC70 cell growth in a cell viability assay, IC50 = 1.05003 μM. | SANGER | |||
| LK-2 | Growth inhibition assay | Inhibition of human LK-2 cell growth in a cell viability assay, IC50 = 1.06014 μM. | SANGER | |||
| LS-411N | Growth inhibition assay | Inhibition of human LS-411N cell growth in a cell viability assay, IC50 = 1.10406 μM. | SANGER | |||
| KYSE-140 | Growth inhibition assay | Inhibition of human KYSE-140 cell growth in a cell viability assay, IC50 = 1.10828 μM. | SANGER | |||
| PSN1 | Growth inhibition assay | Inhibition of human PSN1 cell growth in a cell viability assay, IC50 = 1.11197 μM. | SANGER | |||
| MRK-nu-1 | Growth inhibition assay | Inhibition of human MRK-nu-1 cell growth in a cell viability assay, IC50 = 1.11762 μM. | SANGER | |||
| ACHN | Growth inhibition assay | Inhibition of human ACHN cell growth in a cell viability assay, IC50 = 1.11868 μM. | SANGER | |||
| LB771-HNC | Growth inhibition assay | Inhibition of human LB771-HNC cell growth in a cell viability assay, IC50 = 1.12855 μM. | SANGER | |||
| G-361 | Growth inhibition assay | Inhibition of human G-361 cell growth in a cell viability assay, IC50 = 1.13894 μM. | SANGER | |||
| 639-V | Growth inhibition assay | Inhibition of human 639-V cell growth in a cell viability assay, IC50 = 1.19344 μM. | SANGER | |||
| H4 | Growth inhibition assay | Inhibition of human H4 cell growth in a cell viability assay, IC50 = 1.21951 μM. | SANGER | |||
| RKO | Growth inhibition assay | Inhibition of human RKO cell growth in a cell viability assay, IC50 = 1.22538 μM. | SANGER | |||
| CAL-85-1 | Growth inhibition assay | Inhibition of human CAL-85-1 cell growth in a cell viability assay, IC50 = 1.24589 μM. | SANGER | |||
| ST486 | Growth inhibition assay | Inhibition of human ST486 cell growth in a cell viability assay, IC50 = 1.25737 μM. | SANGER | |||
| SW1783 | Growth inhibition assay | Inhibition of human SW1783 cell growth in a cell viability assay, IC50 = 1.2603 μM. | SANGER | |||
| Calu-6 | Growth inhibition assay | Inhibition of human Calu-6 cell growth in a cell viability assay, IC50 = 1.27529 μM. | SANGER | |||
| ES4 | Growth inhibition assay | Inhibition of human ES4 cell growth in a cell viability assay, IC50 = 1.27763 μM. | SANGER | |||
| NCCIT | Growth inhibition assay | Inhibition of human NCCIT cell growth in a cell viability assay, IC50 = 1.27948 μM. | SANGER | |||
| 697 | Growth inhibition assay | Inhibition of human 697 cell growth in a cell viability assay, IC50 = 1.2829 μM. | SANGER | |||
| RPMI-8402 | Growth inhibition assay | Inhibition of human RPMI-8402 cell growth in a cell viability assay, IC50 = 1.28353 μM. | SANGER | |||
| SH-4 | Growth inhibition assay | Inhibition of human SH-4 cell growth in a cell viability assay, IC50 = 1.30489 μM. | SANGER | |||
| BCPAP | Growth inhibition assay | Inhibition of human BCPAP cell growth in a cell viability assay, IC50 = 1.31707 μM. | SANGER | |||
| HGC-27 | Growth inhibition assay | Inhibition of human HGC-27 cell growth in a cell viability assay, IC50 = 1.31764 μM. | SANGER | |||
| J-RT3-T3-5 | Growth inhibition assay | Inhibition of human J-RT3-T3-5 cell growth in a cell viability assay, IC50 = 1.32093 μM. | SANGER | |||
| SCC-4 | Growth inhibition assay | Inhibition of human SCC-4 cell growth in a cell viability assay, IC50 = 1.32678 μM. | SANGER | |||
| PA-1 | Growth inhibition assay | Inhibition of human PA-1 cell growth in a cell viability assay, IC50 = 1.33654 μM. | SANGER | |||
| SW626 | Growth inhibition assay | Inhibition of human SW626 cell growth in a cell viability assay, IC50 = 1.37341 μM. | SANGER | |||
| CHP-212 | Growth inhibition assay | Inhibition of human CHP-212 cell growth in a cell viability assay, IC50 = 1.37865 μM. | SANGER | |||
| KYSE-70 | Growth inhibition assay | Inhibition of human KYSE-70 cell growth in a cell viability assay, IC50 = 1.40887 μM. | SANGER | |||
| D-542MG | Growth inhibition assay | Inhibition of human D-542MG cell growth in a cell viability assay, IC50 = 1.40979 μM. | SANGER | |||
| GP5d | Growth inhibition assay | Inhibition of human GP5d cell growth in a cell viability assay, IC50 = 1.42296 μM. | SANGER | |||
| MKN28 | Growth inhibition assay | Inhibition of human MKN28 cell growth in a cell viability assay, IC50 = 1.43789 μM. | SANGER | |||
| HT-144 | Growth inhibition assay | Inhibition of human HT-144 cell growth in a cell viability assay, IC50 = 1.4459 μM. | SANGER | |||
| MFE-296 | Growth inhibition assay | Inhibition of human MFE-296 cell growth in a cell viability assay, IC50 = 1.44806 μM. | SANGER | |||
| SW872 | Growth inhibition assay | Inhibition of human SW872 cell growth in a cell viability assay, IC50 = 1.451 μM. | SANGER | |||
| ACN | Growth inhibition assay | Inhibition of human ACN cell growth in a cell viability assay, IC50 = 1.46084 μM. | SANGER | |||
| ONS-76 | Growth inhibition assay | Inhibition of human ONS-76 cell growth in a cell viability assay, IC50 = 1.46712 μM. | SANGER | |||
| ES7 | Growth inhibition assay | Inhibition of human ES7 cell growth in a cell viability assay, IC50 = 1.47409 μM. | SANGER | |||
| LC-2-ad | Growth inhibition assay | Inhibition of human LC-2-ad cell growth in a cell viability assay, IC50 = 1.48847 μM. | SANGER | |||
| IGR-1 | Growth inhibition assay | Inhibition of human IGR-1 cell growth in a cell viability assay, IC50 = 1.6246 μM. | SANGER | |||
| NCI-H810 | Growth inhibition assay | Inhibition of human NCI-H810 cell growth in a cell viability assay, IC50 = 1.62905 μM. | SANGER | |||
| CCRF-CEM | Growth inhibition assay | Inhibition of human CCRF-CEM cell growth in a cell viability assay, IC50 = 1.63988 μM. | SANGER | |||
| YT | Growth inhibition assay | Inhibition of human YT cell growth in a cell viability assay, IC50 = 1.64849 μM. | SANGER | |||
| DoTc2-4510 | Growth inhibition assay | Inhibition of human DoTc2-4510 cell growth in a cell viability assay, IC50 = 1.67753 μM. | SANGER | |||
| TE-11 | Growth inhibition assay | Inhibition of human TE-11 cell growth in a cell viability assay, IC50 = 1.69171 μM. | SANGER | |||
| SK-MEL-24 | Growth inhibition assay | Inhibition of human SK-MEL-24 cell growth in a cell viability assay, IC50 = 1.71207 μM. | SANGER | |||
| PF-382 | Growth inhibition assay | Inhibition of human PF-382 cell growth in a cell viability assay, IC50 = 1.7144 μM. | SANGER | |||
| HC-1 | Growth inhibition assay | Inhibition of human HC-1 cell growth in a cell viability assay, IC50 = 1.75149 μM. | SANGER | |||
| EFM-19 | Growth inhibition assay | Inhibition of human EFM-19 cell growth in a cell viability assay, IC50 = 1.75309 μM. | SANGER | |||
| ChaGo-K-1 | Growth inhibition assay | Inhibition of human ChaGo-K-1 cell growth in a cell viability assay, IC50 = 1.75474 μM. | SANGER | |||
| CAL-62 | Growth inhibition assay | Inhibition of human CAL-62 cell growth in a cell viability assay, IC50 = 1.78729 μM. | SANGER | |||
| SR | Growth inhibition assay | Inhibition of human SR cell growth in a cell viability assay, IC50 = 1.79096 μM. | SANGER | |||
| AU565 | Growth inhibition assay | Inhibition of human AU565 cell growth in a cell viability assay, IC50 = 1.79489 μM. | SANGER | |||
| NCI-H596 | Growth inhibition assay | Inhibition of human NCI-H596 cell growth in a cell viability assay, IC50 = 1.82252 μM. | SANGER | |||
| MG-63 | Growth inhibition assay | Inhibition of human MG-63 cell growth in a cell viability assay, IC50 = 1.8409 μM. | SANGER | |||
| A101D | Growth inhibition assay | Inhibition of human A101D cell growth in a cell viability assay, IC50 = 1.86624 μM. | SANGER | |||
| MKN1 | Growth inhibition assay | Inhibition of human MKN1 cell growth in a cell viability assay, IC50 = 1.87736 μM. | SANGER | |||
| BFTC-905 | Growth inhibition assay | Inhibition of human BFTC-905 cell growth in a cell viability assay, IC50 = 1.88014 μM. | SANGER | |||
| MZ2-MEL | Growth inhibition assay | Inhibition of human MZ2-MEL cell growth in a cell viability assay, IC50 = 1.90376 μM. | SANGER | |||
| SK-MES-1 | Growth inhibition assay | Inhibition of human SK-MES-1 cell growth in a cell viability assay, IC50 = 1.91486 μM. | SANGER | |||
| NY | Growth inhibition assay | Inhibition of human NY cell growth in a cell viability assay, IC50 = 1.92698 μM. | SANGER | |||
| RS4-11 | Growth inhibition assay | Inhibition of human RS4-11 cell growth in a cell viability assay, IC50 = 1.9436 μM. | SANGER | |||
| BL-70 | Growth inhibition assay | Inhibition of human BL-70 cell growth in a cell viability assay, IC50 = 1.95359 μM. | SANGER | |||
| ES1 | Growth inhibition assay | Inhibition of human ES1 cell growth in a cell viability assay, IC50 = 1.96299 μM. | SANGER | |||
| CESS | Growth inhibition assay | Inhibition of human CESS cell growth in a cell viability assay, IC50 = 2.01961 μM. | SANGER | |||
| OCI-AML2 | Growth inhibition assay | Inhibition of human OCI-AML2 cell growth in a cell viability assay, IC50 = 2.03209 μM. | SANGER | |||
| Mewo | Growth inhibition assay | Inhibition of human Mewo cell growth in a cell viability assay, IC50 = 2.03677 μM. | SANGER | |||
| LB2518-MEL | Growth inhibition assay | Inhibition of human LB2518-MEL cell growth in a cell viability assay, IC50 = 2.09909 μM. | SANGER | |||
| G-402 | Growth inhibition assay | Inhibition of human G-402 cell growth in a cell viability assay, IC50 = 2.10728 μM. | SANGER | |||
| LS-123 | Growth inhibition assay | Inhibition of human LS-123 cell growth in a cell viability assay, IC50 = 2.1086 μM. | SANGER | |||
| MDA-MB-361 | Growth inhibition assay | Inhibition of human MDA-MB-361 cell growth in a cell viability assay, IC50 = 2.11443 μM. | SANGER | |||
| NOS-1 | Growth inhibition assay | Inhibition of human NOS-1 cell growth in a cell viability assay, IC50 = 2.13063 μM. | SANGER | |||
| 8-MG-BA | Growth inhibition assay | Inhibition of human 8-MG-BA cell growth in a cell viability assay, IC50 = 2.13374 μM. | SANGER | |||
| SW962 | Growth inhibition assay | Inhibition of human SW962 cell growth in a cell viability assay, IC50 = 2.15391 μM. | SANGER | |||
| SF295 | Growth inhibition assay | Inhibition of human SF295 cell growth in a cell viability assay, IC50 = 2.16427 μM. | SANGER | |||
| DU-4475 | Growth inhibition assay | Inhibition of human DU-4475 cell growth in a cell viability assay, IC50 = 2.18699 μM. | SANGER | |||
| OVCAR-4 | Growth inhibition assay | Inhibition of human OVCAR-4 cell growth in a cell viability assay, IC50 = 2.2119 μM. | SANGER | |||
| NCI-H520 | Growth inhibition assay | Inhibition of human NCI-H520 cell growth in a cell viability assay, IC50 = 2.22253 μM. | SANGER | |||
| MEL-HO | Growth inhibition assay | Inhibition of human MEL-HO cell growth in a cell viability assay, IC50 = 2.23646 μM. | SANGER | |||
| BPH-1 | Growth inhibition assay | Inhibition of human BPH-1 cell growth in a cell viability assay, IC50 = 2.2449 μM. | SANGER | |||
| NCI-H2087 | Growth inhibition assay | Inhibition of human NCI-H2087 cell growth in a cell viability assay, IC50 = 2.276 μM. | SANGER | |||
| LCLC-97TM1 | Growth inhibition assay | Inhibition of human LCLC-97TM1 cell growth in a cell viability assay, IC50 = 2.29912 μM. | SANGER | |||
| 647-V | Growth inhibition assay | Inhibition of human 647-V cell growth in a cell viability assay, IC50 = 2.30455 μM. | SANGER | |||
| KE-37 | Growth inhibition assay | Inhibition of human KE-37 cell growth in a cell viability assay, IC50 = 2.31682 μM. | SANGER | |||
| NCI-H2030 | Growth inhibition assay | Inhibition of human NCI-H2030 cell growth in a cell viability assay, IC50 = 2.34549 μM. | SANGER | |||
| VA-ES-BJ | Growth inhibition assay | Inhibition of human VA-ES-BJ cell growth in a cell viability assay, IC50 = 2.36492 μM. | SANGER | |||
| BV-173 | Growth inhibition assay | Inhibition of human BV-173 cell growth in a cell viability assay, IC50 = 2.38298 μM. | SANGER | |||
| CA46 | Growth inhibition assay | Inhibition of human CA46 cell growth in a cell viability assay, IC50 = 2.39332 μM. | SANGER | |||
| NCI-H522 | Growth inhibition assay | Inhibition of human NCI-H522 cell growth in a cell viability assay, IC50 = 2.39659 μM. | SANGER | |||
| LS-1034 | Growth inhibition assay | Inhibition of human LS-1034 cell growth in a cell viability assay, IC50 = 2.45884 μM. | SANGER | |||
| SK-OV-3 | Growth inhibition assay | Inhibition of human SK-OV-3 cell growth in a cell viability assay, IC50 = 2.49065 μM. | SANGER | |||
| Daudi | Growth inhibition assay | Inhibition of human Daudi cell growth in a cell viability assay, IC50 = 2.50076 μM. | SANGER | |||
| ATN-1 | Growth inhibition assay | Inhibition of human ATN-1 cell growth in a cell viability assay, IC50 = 2.53822 μM. | SANGER | |||
| LC4-1 | Growth inhibition assay | Inhibition of human LC4-1 cell growth in a cell viability assay, IC50 = 2.54983 μM. | SANGER | |||
| NCI-H1648 | Growth inhibition assay | Inhibition of human NCI-H1648 cell growth in a cell viability assay, IC50 = 2.55881 μM. | SANGER | |||
| HCC1569 | Growth inhibition assay | Inhibition of human HCC1569 cell growth in a cell viability assay, IC50 = 2.58239 μM. | SANGER | |||
| TE-1 | Growth inhibition assay | Inhibition of human TE-1 cell growth in a cell viability assay, IC50 = 2.60619 μM. | SANGER | |||
| HSC-3 | Growth inhibition assay | Inhibition of human HSC-3 cell growth in a cell viability assay, IC50 = 2.63027 μM. | SANGER | |||
| BB65-RCC | Growth inhibition assay | Inhibition of human BB65-RCC cell growth in a cell viability assay, IC50 = 2.63834 μM. | SANGER | |||
| LB831-BLC | Growth inhibition assay | Inhibition of human LB831-BLC cell growth in a cell viability assay, IC50 = 2.64444 μM. | SANGER | |||
| NCI-H747 | Growth inhibition assay | Inhibition of human NCI-H747 cell growth in a cell viability assay, IC50 = 2.65802 μM. | SANGER | |||
| EB2 | Growth inhibition assay | Inhibition of human EB2 cell growth in a cell viability assay, IC50 = 2.71829 μM. | SANGER | |||
| RH-1 | Growth inhibition assay | Inhibition of human RH-1 cell growth in a cell viability assay, IC50 = 2.74912 μM. | SANGER | |||
| MC-IXC | Growth inhibition assay | Inhibition of human MC-IXC cell growth in a cell viability assay, IC50 = 2.76192 μM. | SANGER | |||
| BL-41 | Growth inhibition assay | Inhibition of human BL-41 cell growth in a cell viability assay, IC50 = 2.77315 μM. | SANGER | |||
| MKN45 | Growth inhibition assay | Inhibition of human MKN45 cell growth in a cell viability assay, IC50 = 2.78805 μM. | SANGER | |||
| SBC-5 | Growth inhibition assay | Inhibition of human SBC-5 cell growth in a cell viability assay, IC50 = 2.81094 μM. | SANGER | |||
| TE-12 | Growth inhibition assay | Inhibition of human TE-12 cell growth in a cell viability assay, IC50 = 2.81604 μM. | SANGER | |||
| IST-MEL1 | Growth inhibition assay | Inhibition of human IST-MEL1 cell growth in a cell viability assay, IC50 = 2.81622 μM. | SANGER | |||
| MZ1-PC | Growth inhibition assay | Inhibition of human MZ1-PC cell growth in a cell viability assay, IC50 = 2.82459 μM. | SANGER | |||
| GR-ST | Growth inhibition assay | Inhibition of human GR-ST cell growth in a cell viability assay, IC50 = 2.87457 μM. | SANGER | |||
| SW954 | Growth inhibition assay | Inhibition of human SW954 cell growth in a cell viability assay, IC50 = 2.90092 μM. | SANGER | |||
| Ca9-22 | Growth inhibition assay | Inhibition of human Ca9-22 cell growth in a cell viability assay, IC50 = 2.93767 μM. | SANGER | |||
| BC-3 | Growth inhibition assay | Inhibition of human BC-3 cell growth in a cell viability assay, IC50 = 2.97824 μM. | SANGER | |||
| GT3TKB | Growth inhibition assay | Inhibition of human GT3TKB cell growth in a cell viability assay, IC50 = 3.00817 μM. | SANGER | |||
| HuP-T4 | Growth inhibition assay | Inhibition of human HuP-T4 cell growth in a cell viability assay, IC50 = 3.02832 μM. | SANGER | |||
| HCE-4 | Growth inhibition assay | Inhibition of human HCE-4 cell growth in a cell viability assay, IC50 = 3.0403 μM. | SANGER | |||
| KG-1 | Growth inhibition assay | Inhibition of human KG-1 cell growth in a cell viability assay, IC50 = 3.06496 μM. | SANGER | |||
| BHT-101 | Growth inhibition assay | Inhibition of human BHT-101 cell growth in a cell viability assay, IC50 = 3.08607 μM. | SANGER | |||
| CAL-27 | Growth inhibition assay | Inhibition of human CAL-27 cell growth in a cell viability assay, IC50 = 3.09821 μM. | SANGER | |||
| VMRC-RCZ | Growth inhibition assay | Inhibition of human VMRC-RCZ cell growth in a cell viability assay, IC50 = 3.18272 μM. | SANGER | |||
| HOP-62 | Growth inhibition assay | Inhibition of human HOP-62 cell growth in a cell viability assay, IC50 = 3.20611 μM. | SANGER | |||
| ABC-1 | Growth inhibition assay | Inhibition of human ABC-1 cell growth in a cell viability assay, IC50 = 3.21208 μM. | SANGER | |||
| 22RV1 | Growth inhibition assay | Inhibition of human 22RV1 cell growth in a cell viability assay, IC50 = 3.21924 μM. | SANGER | |||
| MHH-PREB-1 | Growth inhibition assay | Inhibition of human MHH-PREB-1 cell growth in a cell viability assay, IC50 = 3.29969 μM. | SANGER | |||
| A2780 | Growth inhibition assay | Inhibition of human A2780 cell growth in a cell viability assay, IC50 = 3.3048 μM. | SANGER | |||
| MONO-MAC-6 | Growth inhibition assay | Inhibition of human MONO-MAC-6 cell growth in a cell viability assay, IC50 = 3.34894 μM. | SANGER | |||
| KYSE-180 | Growth inhibition assay | Inhibition of human KYSE-180 cell growth in a cell viability assay, IC50 = 3.36908 μM. | SANGER | |||
| COLO-668 | Growth inhibition assay | Inhibition of human COLO-668 cell growth in a cell viability assay, IC50 = 3.3922 μM. | SANGER | |||
| KYSE-410 | Growth inhibition assay | Inhibition of human KYSE-410 cell growth in a cell viability assay, IC50 = 3.5007 μM. | SANGER | |||
| CAL-51 | Growth inhibition assay | Inhibition of human CAL-51 cell growth in a cell viability assay, IC50 = 3.52671 μM. | SANGER | |||
| NCI-H2171 | Growth inhibition assay | Inhibition of human NCI-H2171 cell growth in a cell viability assay, IC50 = 3.54602 μM. | SANGER | |||
| SK-LU-1 | Growth inhibition assay | Inhibition of human SK-LU-1 cell growth in a cell viability assay, IC50 = 3.54814 μM. | SANGER | |||
| A204 | Growth inhibition assay | Inhibition of human A204 cell growth in a cell viability assay, IC50 = 3.58082 μM. | SANGER | |||
| EW-16 | Growth inhibition assay | Inhibition of human EW-16 cell growth in a cell viability assay, IC50 = 3.59334 μM. | SANGER | |||
| SW13 | Growth inhibition assay | Inhibition of human SW13 cell growth in a cell viability assay, IC50 = 3.60153 μM. | SANGER | |||
| MEG-01 | Growth inhibition assay | Inhibition of human MEG-01 cell growth in a cell viability assay, IC50 = 3.61342 μM. | SANGER | |||
| CAL-33 | Growth inhibition assay | Inhibition of human CAL-33 cell growth in a cell viability assay, IC50 = 3.67639 μM. | SANGER | |||
| GB-1 | Growth inhibition assay | Inhibition of human GB-1 cell growth in a cell viability assay, IC50 = 3.70058 μM. | SANGER | |||
| SK-MEL-30 | Growth inhibition assay | Inhibition of human SK-MEL-30 cell growth in a cell viability assay, IC50 = 3.74333 μM. | SANGER | |||
| GAK | Growth inhibition assay | Inhibition of human GAK cell growth in a cell viability assay, IC50 = 3.80232 μM. | SANGER | |||
| SF126 | Growth inhibition assay | Inhibition of human SF126 cell growth in a cell viability assay, IC50 = 3.87725 μM. | SANGER | |||
| U-698-M | Growth inhibition assay | Inhibition of human U-698-M cell growth in a cell viability assay, IC50 = 3.99076 μM. | SANGER | |||
| no-11 | Growth inhibition assay | Inhibition of human no-11 cell growth in a cell viability assay, IC50 = 4.00889 μM. | SANGER | |||
| MC116 | Growth inhibition assay | Inhibition of human MC116 cell growth in a cell viability assay, IC50 = 4.03257 μM. | SANGER | |||
| Detroit562 | Growth inhibition assay | Inhibition of human Detroit562 cell growth in a cell viability assay, IC50 = 4.06658 μM. | SANGER | |||
| HOS | Growth inhibition assay | Inhibition of human HOS cell growth in a cell viability assay, IC50 = 4.09632 μM. | SANGER | |||
| LXF-289 | Growth inhibition assay | Inhibition of human LXF-289 cell growth in a cell viability assay, IC50 = 4.12623 μM. | SANGER | |||
| HuO-3N1 | Growth inhibition assay | Inhibition of human HuO-3N1 cell growth in a cell viability assay, IC50 = 4.17121 μM. | SANGER | |||
| COLO-205 | Growth inhibition assay | Inhibition of human COLO-205 cell growth in a cell viability assay, IC50 = 4.17864 μM. | SANGER | |||
| J82 | Growth inhibition assay | Inhibition of human J82 cell growth in a cell viability assay, IC50 = 4.18416 μM. | SANGER | |||
| NUGC-3 | Growth inhibition assay | Inhibition of human NUGC-3 cell growth in a cell viability assay, IC50 = 4.18686 μM. | SANGER | |||
| 769-P | Growth inhibition assay | Inhibition of human 769-P cell growth in a cell viability assay, IC50 = 4.19237 μM. | SANGER | |||
| EFO-21 | Growth inhibition assay | Inhibition of human EFO-21 cell growth in a cell viability assay, IC50 = 4.26199 μM. | SANGER | |||
| HuCCT1 | Growth inhibition assay | Inhibition of human HuCCT1 cell growth in a cell viability assay, IC50 = 4.31306 μM. | SANGER | |||
| BxPC-3 | Growth inhibition assay | Inhibition of human BxPC-3 cell growth in a cell viability assay, IC50 = 4.3263 μM. | SANGER | |||
| GI-1 | Growth inhibition assay | Inhibition of human GI-1 cell growth in a cell viability assay, IC50 = 4.42376 μM. | SANGER | |||
| Calu-3 | Growth inhibition assay | Inhibition of human Calu-3 cell growth in a cell viability assay, IC50 = 4.429 μM. | SANGER | |||
| OVCAR-8 | Growth inhibition assay | Inhibition of human OVCAR-8 cell growth in a cell viability assay, IC50 = 4.4502 μM. | SANGER | |||
| C-33-A | Growth inhibition assay | Inhibition of human C-33-A cell growth in a cell viability assay, IC50 = 4.49182 μM. | SANGER | |||
| AGS | Growth inhibition assay | Inhibition of human AGS cell growth in a cell viability assay, IC50 = 4.53323 μM. | SANGER | |||
| CTV-1 | Growth inhibition assay | Inhibition of human CTV-1 cell growth in a cell viability assay, IC50 = 4.57803 μM. | SANGER | |||
| EFE-184 | Growth inhibition assay | Inhibition of human EFE-184 cell growth in a cell viability assay, IC50 = 4.5793 μM. | SANGER | |||
| MES-SA | Growth inhibition assay | Inhibition of human MES-SA cell growth in a cell viability assay, IC50 = 4.61106 μM. | SANGER | |||
| NB13 | Growth inhibition assay | Inhibition of human NB13 cell growth in a cell viability assay, IC50 = 4.62248 μM. | SANGER | |||
| C3A | Growth inhibition assay | Inhibition of human C3A cell growth in a cell viability assay, IC50 = 4.64119 μM. | SANGER | |||
| HCC1419 | Growth inhibition assay | Inhibition of human HCC1419 cell growth in a cell viability assay, IC50 = 4.64795 μM. | SANGER | |||
| SK-N-DZ | Growth inhibition assay | Inhibition of human SK-N-DZ cell growth in a cell viability assay, IC50 = 4.70596 μM. | SANGER | |||
| KURAMOCHI | Growth inhibition assay | Inhibition of human KURAMOCHI cell growth in a cell viability assay, IC50 = 4.77799 μM. | SANGER | |||
| K-562 | Growth inhibition assay | Inhibition of human K-562 cell growth in a cell viability assay, IC50 = 4.82317 μM. | SANGER | |||
| HTC-C3 | Growth inhibition assay | Inhibition of human HTC-C3 cell growth in a cell viability assay, IC50 = 4.82962 μM. | SANGER | |||
| L-540 | Growth inhibition assay | Inhibition of human L-540 cell growth in a cell viability assay, IC50 = 4.83033 μM. | SANGER | |||
| SK-UT-1 | Growth inhibition assay | Inhibition of human SK-UT-1 cell growth in a cell viability assay, IC50 = 4.86141 μM. | SANGER | |||
| HCT-116 | Growth inhibition assay | Inhibition of human HCT-116 cell growth in a cell viability assay, IC50 = 4.87587 μM. | SANGER | |||
| MOLT-4 | Growth inhibition assay | Inhibition of human MOLT-4 cell growth in a cell viability assay, IC50 = 4.9073 μM. | SANGER | |||
| NCI-H358 | Growth inhibition assay | Inhibition of human NCI-H358 cell growth in a cell viability assay, IC50 = 4.91507 μM. | SANGER | |||
| NCI-H460 | Growth inhibition assay | Inhibition of human NCI-H460 cell growth in a cell viability assay, IC50 = 4.92762 μM. | SANGER | |||
| BE-13 | Growth inhibition assay | Inhibition of human BE-13 cell growth in a cell viability assay, IC50 = 4.93538 μM. | SANGER | |||
| CAL-72 | Growth inhibition assay | Inhibition of human CAL-72 cell growth in a cell viability assay, IC50 = 4.95398 μM. | SANGER | |||
| NCI-H2228 | Growth inhibition assay | Inhibition of human NCI-H2228 cell growth in a cell viability assay, IC50 = 4.97741 μM. | SANGER | |||
| SK-MEL-3 | Growth inhibition assay | Inhibition of human SK-MEL-3 cell growth in a cell viability assay, IC50 = 4.97767 μM. | SANGER | |||
| CGTH-W-1 | Growth inhibition assay | Inhibition of human CGTH-W-1 cell growth in a cell viability assay, IC50 = 5.0072 μM. | SANGER | |||
| PANC-10-05 | Growth inhibition assay | Inhibition of human PANC-10-05 cell growth in a cell viability assay, IC50 = 5.05437 μM. | SANGER | |||
| KMOE-2 | Growth inhibition assay | Inhibition of human KMOE-2 cell growth in a cell viability assay, IC50 = 5.17561 μM. | SANGER | |||
| HSC-2 | Growth inhibition assay | Inhibition of human HSC-2 cell growth in a cell viability assay, IC50 = 5.2025 μM. | SANGER | |||
| MHH-ES-1 | Growth inhibition assay | Inhibition of human MHH-ES-1 cell growth in a cell viability assay, IC50 = 5.26504 μM. | SANGER | |||
| BEN | Growth inhibition assay | Inhibition of human BEN cell growth in a cell viability assay, IC50 = 5.39709 μM. | SANGER | |||
| NH-12 | Growth inhibition assay | Inhibition of human NH-12 cell growth in a cell viability assay, IC50 = 5.58792 μM. | SANGER | |||
| COLO-680N | Growth inhibition assay | Inhibition of human COLO-680N cell growth in a cell viability assay, IC50 = 5.62717 μM. | SANGER | |||
| TK10 | Growth inhibition assay | Inhibition of human TK10 cell growth in a cell viability assay, IC50 = 5.67933 μM. | SANGER | |||
| COLO-684 | Growth inhibition assay | Inhibition of human COLO-684 cell growth in a cell viability assay, IC50 = 5.71062 μM. | SANGER | |||
| PANC-03-27 | Growth inhibition assay | Inhibition of human PANC-03-27 cell growth in a cell viability assay, IC50 = 5.72558 μM. | SANGER | |||
| OE19 | Growth inhibition assay | Inhibition of human OE19 cell growth in a cell viability assay, IC50 = 5.75409 μM. | SANGER | |||
| KNS-42 | Growth inhibition assay | Inhibition of human KNS-42 cell growth in a cell viability assay, IC50 = 5.76212 μM. | SANGER | |||
| VM-CUB-1 | Growth inhibition assay | Inhibition of human VM-CUB-1 cell growth in a cell viability assay, IC50 = 5.76408 μM. | SANGER | |||
| TCCSUP | Growth inhibition assay | Inhibition of human TCCSUP cell growth in a cell viability assay, IC50 = 5.80186 μM. | SANGER | |||
| IPC-298 | Growth inhibition assay | Inhibition of human IPC-298 cell growth in a cell viability assay, IC50 = 5.82247 μM. | SANGER | |||
| MDA-MB-231 | Growth inhibition assay | Inhibition of human MDA-MB-231 cell growth in a cell viability assay, IC50 = 5.82732 μM. | SANGER | |||
| BB49-HNC | Growth inhibition assay | Inhibition of human BB49-HNC cell growth in a cell viability assay, IC50 = 5.8599 μM. | SANGER | |||
| NCI-H1573 | Growth inhibition assay | Inhibition of human NCI-H1573 cell growth in a cell viability assay, IC50 = 5.90435 μM. | SANGER | |||
| SCC-25 | Growth inhibition assay | Inhibition of human SCC-25 cell growth in a cell viability assay, IC50 = 5.91345 μM. | SANGER | |||
| HuH-7 | Growth inhibition assay | Inhibition of human HuH-7 cell growth in a cell viability assay, IC50 = 6.05076 μM. | SANGER | |||
| HCC38 | Growth inhibition assay | Inhibition of human HCC38 cell growth in a cell viability assay, IC50 = 6.10436 μM. | SANGER | |||
| C-4-II | Growth inhibition assay | Inhibition of human C-4-II cell growth in a cell viability assay, IC50 = 6.14144 μM. | SANGER | |||
| LB2241-RCC | Growth inhibition assay | Inhibition of human LB2241-RCC cell growth in a cell viability assay, IC50 = 6.16331 μM. | SANGER | |||
| KYSE-150 | Growth inhibition assay | Inhibition of human KYSE-150 cell growth in a cell viability assay, IC50 = 6.2004 μM. | SANGER | |||
| Becker | Growth inhibition assay | Inhibition of human Becker cell growth in a cell viability assay, IC50 = 6.40382 μM. | SANGER | |||
| LAMA-84 | Growth inhibition assay | Inhibition of human LAMA-84 cell growth in a cell viability assay, IC50 = 6.41967 μM. | SANGER | |||
| EW-13 | Growth inhibition assay | Inhibition of human EW-13 cell growth in a cell viability assay, IC50 = 6.47692 μM. | SANGER | |||
| OMC-1 | Growth inhibition assay | Inhibition of human OMC-1 cell growth in a cell viability assay, IC50 = 6.491 μM. | SANGER | |||
| COLO-741 | Growth inhibition assay | Inhibition of human COLO-741 cell growth in a cell viability assay, IC50 = 6.54892 μM. | SANGER | |||
| 23132-87 | Growth inhibition assay | Inhibition of human 23132-87 cell growth in a cell viability assay, IC50 = 6.70181 μM. | SANGER | |||
| EoL-1-cell | Growth inhibition assay | Inhibition of human EoL-1-cell cell growth in a cell viability assay, IC50 = 6.71228 μM. | SANGER | |||
| HAL-01 | Growth inhibition assay | Inhibition of human HAL-01 cell growth in a cell viability assay, IC50 = 6.73991 μM. | SANGER | |||
| HL-60 | Growth inhibition assay | Inhibition of human HL-60 cell growth in a cell viability assay, IC50 = 6.929 μM. | SANGER | |||
| SNU-387 | Growth inhibition assay | Inhibition of human SNU-387 cell growth in a cell viability assay, IC50 = 7.05531 μM. | SANGER | |||
| LU-99A | Growth inhibition assay | Inhibition of human LU-99A cell growth in a cell viability assay, IC50 = 7.09065 μM. | SANGER | |||
| U251 | Growth inhibition assay | Inhibition of human U251 cell growth in a cell viability assay, IC50 = 7.09105 μM. | SANGER | |||
| HEC-1 | Growth inhibition assay | Inhibition of human HEC-1 cell growth in a cell viability assay, IC50 = 7.15107 μM. | SANGER | |||
| TE-6 | Growth inhibition assay | Inhibition of human TE-6 cell growth in a cell viability assay, IC50 = 7.20609 μM. | SANGER | |||
| COR-L105 | Growth inhibition assay | Inhibition of human COR-L105 cell growth in a cell viability assay, IC50 = 7.23614 μM. | SANGER | |||
| SU-DHL-1 | Growth inhibition assay | Inhibition of human SU-DHL-1 cell growth in a cell viability assay, IC50 = 7.26618 μM. | SANGER | |||
| NCI-H661 | Growth inhibition assay | Inhibition of human NCI-H661 cell growth in a cell viability assay, IC50 = 7.49639 μM. | SANGER | |||
| HPAF-II | Growth inhibition assay | Inhibition of human HPAF-II cell growth in a cell viability assay, IC50 = 7.57285 μM. | SANGER | |||
| HCC1187 | Growth inhibition assay | Inhibition of human HCC1187 cell growth in a cell viability assay, IC50 = 7.6136 μM. | SANGER | |||
| LB996-RCC | Growth inhibition assay | Inhibition of human LB996-RCC cell growth in a cell viability assay, IC50 = 7.69178 μM. | SANGER | |||
| CTB-1 | Growth inhibition assay | Inhibition of human CTB-1 cell growth in a cell viability assay, IC50 = 7.81408 μM. | SANGER | |||
| DOHH-2 | Growth inhibition assay | Inhibition of human DOHH-2 cell growth in a cell viability assay, IC50 = 7.9087 μM. | SANGER | |||
| COR-L279 | Growth inhibition assay | Inhibition of human COR-L279 cell growth in a cell viability assay, IC50 = 7.95449 μM. | SANGER | |||
| NB12 | Growth inhibition assay | Inhibition of human NB12 cell growth in a cell viability assay, IC50 = 7.98947 μM. | SANGER | |||
| HCE-T | Growth inhibition assay | Inhibition of human HCE-T cell growth in a cell viability assay, IC50 = 7.99856 μM. | SANGER | |||
| SF268 | Growth inhibition assay | Inhibition of human SF268 cell growth in a cell viability assay, IC50 = 8.06996 μM. | SANGER | |||
| NCI-H1437 | Growth inhibition assay | Inhibition of human NCI-H1437 cell growth in a cell viability assay, IC50 = 8.2116 μM. | SANGER | |||
| RPMI-2650 | Growth inhibition assay | Inhibition of human RPMI-2650 cell growth in a cell viability assay, IC50 = 8.28546 μM. | SANGER | |||
| HT | Growth inhibition assay | Inhibition of human HT cell growth in a cell viability assay, IC50 = 8.29045 μM. | SANGER | |||
| BFTC-909 | Growth inhibition assay | Inhibition of human BFTC-909 cell growth in a cell viability assay, IC50 = 8.37244 μM. | SANGER | |||
| FADU | Growth inhibition assay | Inhibition of human FADU cell growth in a cell viability assay, IC50 = 8.38402 μM. | SANGER | |||
| NCI-H2170 | Growth inhibition assay | Inhibition of human NCI-H2170 cell growth in a cell viability assay, IC50 = 8.38671 μM. | SANGER | |||
| RCC10RGB | Growth inhibition assay | Inhibition of human RCC10RGB cell growth in a cell viability assay, IC50 = 8.48795 μM. | SANGER | |||
| DSH1 | Growth inhibition assay | Inhibition of human DSH1 cell growth in a cell viability assay, IC50 = 8.52998 μM. | SANGER | |||
| SW1088 | Growth inhibition assay | Inhibition of human SW1088 cell growth in a cell viability assay, IC50 = 8.56921 μM. | SANGER | |||
| JEG-3 | Growth inhibition assay | Inhibition of human JEG-3 cell growth in a cell viability assay, IC50 = 8.60589 μM. | SANGER | |||
| SW48 | Growth inhibition assay | Inhibition of human SW48 cell growth in a cell viability assay, IC50 = 8.61062 μM. | SANGER | |||
| DU-145 | Growth inhibition assay | Inhibition of human DU-145 cell growth in a cell viability assay, IC50 = 8.6558 μM. | SANGER | |||
| SJRH30 | Growth inhibition assay | Inhibition of human SJRH30 cell growth in a cell viability assay, IC50 = 8.71431 μM. | SANGER | |||
| LB373-MEL-D | Growth inhibition assay | Inhibition of human LB373-MEL-D cell growth in a cell viability assay, IC50 = 8.85273 μM. | SANGER | |||
| KLE | Growth inhibition assay | Inhibition of human KLE cell growth in a cell viability assay, IC50 = 8.91079 μM. | SANGER | |||
| GAMG | Growth inhibition assay | Inhibition of human GAMG cell growth in a cell viability assay, IC50 = 8.91612 μM. | SANGER | |||
| HCC1937 | Growth inhibition assay | Inhibition of human HCC1937 cell growth in a cell viability assay, IC50 = 9.01672 μM. | SANGER | |||
| SNB75 | Growth inhibition assay | Inhibition of human SNB75 cell growth in a cell viability assay, IC50 = 9.02338 μM. | SANGER | |||
| RPMI-8226 | Growth inhibition assay | Inhibition of human RPMI-8226 cell growth in a cell viability assay, IC50 = 9.02854 μM. | SANGER | |||
| MC-CAR | Growth inhibition assay | Inhibition of human MC-CAR cell growth in a cell viability assay, IC50 = 9.07499 μM. | SANGER | |||
| EB-3 | Growth inhibition assay | Inhibition of human EB-3 cell growth in a cell viability assay, IC50 = 9.10261 μM. | SANGER | |||
| NCI-H82 | Growth inhibition assay | Inhibition of human NCI-H82 cell growth in a cell viability assay, IC50 = 9.10469 μM. | SANGER | |||
| HOP-92 | Growth inhibition assay | Inhibition of human HOP-92 cell growth in a cell viability assay, IC50 = 9.13092 μM. | SANGER | |||
| Ca-Ski | Growth inhibition assay | Inhibition of human Ca-Ski cell growth in a cell viability assay, IC50 = 9.17136 μM. | SANGER | |||
| JiyoyeP-2003 | Growth inhibition assay | Inhibition of human JiyoyeP-2003 cell growth in a cell viability assay, IC50 = 9.17954 μM. | SANGER | |||
| NCI-H292 | Growth inhibition assay | Inhibition of human NCI-H292 cell growth in a cell viability assay, IC50 = 9.27777 μM. | SANGER | |||
| S-117 | Growth inhibition assay | Inhibition of human S-117 cell growth in a cell viability assay, IC50 = 9.59474 μM. | SANGER | |||
| NCI-H2405 | Growth inhibition assay | Inhibition of human NCI-H2405 cell growth in a cell viability assay, IC50 = 9.59843 μM. | SANGER | |||
| CP66-MEL | Growth inhibition assay | Inhibition of human CP66-MEL cell growth in a cell viability assay, IC50 = 9.8193 μM. | SANGER | |||
| SW837 | Growth inhibition assay | Inhibition of human SW837 cell growth in a cell viability assay, IC50 = 9.89177 μM. | SANGER | |||
| SK-NEP-1 | Growth inhibition assay | Inhibition of human SK-NEP-1 cell growth in a cell viability assay, IC50 = 9.93648 μM. | SANGER | |||
| SHP-77 | Growth inhibition assay | Inhibition of human SHP-77 cell growth in a cell viability assay, IC50 = 9.9441 μM. | SANGER | |||
| A172 | Growth inhibition assay | Inhibition of human A172 cell growth in a cell viability assay, IC50 = 10.1272 μM. | SANGER | |||
| HT-1080 | Growth inhibition assay | Inhibition of human HT-1080 cell growth in a cell viability assay, IC50 = 10.3244 μM. | SANGER | |||
| P12-ICHIKAWA | Growth inhibition assay | Inhibition of human P12-ICHIKAWA cell growth in a cell viability assay, IC50 = 10.416 μM. | SANGER | |||
| SNU-C2B | Growth inhibition assay | Inhibition of human SNU-C2B cell growth in a cell viability assay, IC50 = 10.4415 μM. | SANGER | |||
| ES8 | Growth inhibition assay | Inhibition of human ES8 cell growth in a cell viability assay, IC50 = 10.4468 μM. | SANGER | |||
| A253 | Growth inhibition assay | Inhibition of human A253 cell growth in a cell viability assay, IC50 = 10.4636 μM. | SANGER | |||
| NCI-H719 | Growth inhibition assay | Inhibition of human NCI-H719 cell growth in a cell viability assay, IC50 = 10.4881 μM. | SANGER | |||
| HO-1-N-1 | Growth inhibition assay | Inhibition of human HO-1-N-1 cell growth in a cell viability assay, IC50 = 10.544 μM. | SANGER | |||
| MHH-CALL-2 | Growth inhibition assay | Inhibition of human MHH-CALL-2 cell growth in a cell viability assay, IC50 = 10.5621 μM. | SANGER | |||
| Capan-2 | Growth inhibition assay | Inhibition of human Capan-2 cell growth in a cell viability assay, IC50 = 10.649 μM. | SANGER | |||
| U031 | Growth inhibition assay | Inhibition of human U031 cell growth in a cell viability assay, IC50 = 10.6516 μM. | SANGER | |||
| DB | Growth inhibition assay | Inhibition of human DB cell growth in a cell viability assay, IC50 = 10.7206 μM. | SANGER | |||
| SCC-15 | Growth inhibition assay | Inhibition of human SCC-15 cell growth in a cell viability assay, IC50 = 10.775 μM. | SANGER | |||
| RT4 | Growth inhibition assay | Inhibition of human RT4 cell growth in a cell viability assay, IC50 = 10.8937 μM. | SANGER | |||
| KNS-81-FD | Growth inhibition assay | Inhibition of human KNS-81-FD cell growth in a cell viability assay, IC50 = 11.1663 μM. | SANGER | |||
| PC-3 | Growth inhibition assay | Inhibition of human PC-3 cell growth in a cell viability assay, IC50 = 11.289 μM. | SANGER | |||
| LNCaP-Clone-FGC | Growth inhibition assay | Inhibition of human LNCaP-Clone-FGC cell growth in a cell viability assay, IC50 = 11.4558 μM. | SANGER | |||
| EGI-1 | Growth inhibition assay | Inhibition of human EGI-1 cell growth in a cell viability assay, IC50 = 11.5579 μM. | SANGER | |||
| HT-1376 | Growth inhibition assay | Inhibition of human HT-1376 cell growth in a cell viability assay, IC50 = 11.5804 μM. | SANGER | |||
| NB10 | Growth inhibition assay | Inhibition of human NB10 cell growth in a cell viability assay, IC50 = 11.6815 μM. | SANGER | |||
| HT-1197 | Growth inhibition assay | Inhibition of human HT-1197 cell growth in a cell viability assay, IC50 = 11.7454 μM. | SANGER | |||
| T98G | Growth inhibition assay | Inhibition of human T98G cell growth in a cell viability assay, IC50 = 11.7966 μM. | SANGER | |||
| A2058 | Growth inhibition assay | Inhibition of human A2058 cell growth in a cell viability assay, IC50 = 11.811 μM. | SANGER | |||
| ES3 | Growth inhibition assay | Inhibition of human ES3 cell growth in a cell viability assay, IC50 = 11.8286 μM. | SANGER | |||
| TE-5 | Growth inhibition assay | Inhibition of human TE-5 cell growth in a cell viability assay, IC50 = 11.862 μM. | SANGER | |||
| CAL-54 | Growth inhibition assay | Inhibition of human CAL-54 cell growth in a cell viability assay, IC50 = 11.9056 μM. | SANGER | |||
| HCC1806 | Growth inhibition assay | Inhibition of human HCC1806 cell growth in a cell viability assay, IC50 = 11.9105 μM. | SANGER | |||
| RD | Growth inhibition assay | Inhibition of human RD cell growth in a cell viability assay, IC50 = 12.0261 μM. | SANGER | |||
| COLO-792 | Growth inhibition assay | Inhibition of human COLO-792 cell growth in a cell viability assay, IC50 = 12.2102 μM. | SANGER | |||
| EW-1 | Growth inhibition assay | Inhibition of human EW-1 cell growth in a cell viability assay, IC50 = 12.2857 μM. | SANGER | |||
| SW1990 | Growth inhibition assay | Inhibition of human SW1990 cell growth in a cell viability assay, IC50 = 12.3978 μM. | SANGER | |||
| GCIY | Growth inhibition assay | Inhibition of human GCIY cell growth in a cell viability assay, IC50 = 12.4816 μM. | SANGER | |||
| Raji | Growth inhibition assay | Inhibition of human Raji cell growth in a cell viability assay, IC50 = 12.4915 μM. | SANGER | |||
| COLO-829 | Growth inhibition assay | Inhibition of human COLO-829 cell growth in a cell viability assay, IC50 = 12.5517 μM. | SANGER | |||
| OE33 | Growth inhibition assay | Inhibition of human OE33 cell growth in a cell viability assay, IC50 = 12.5575 μM. | SANGER | |||
| UM-UC-3 | Growth inhibition assay | Inhibition of human UM-UC-3 cell growth in a cell viability assay, IC50 = 12.6452 μM. | SANGER | |||
| KARPAS-45 | Growth inhibition assay | Inhibition of human KARPAS-45 cell growth in a cell viability assay, IC50 = 12.8701 μM. | SANGER | |||
| SK-CO-1 | Growth inhibition assay | Inhibition of human SK-CO-1 cell growth in a cell viability assay, IC50 = 12.9578 μM. | SANGER | |||
| SN12C | Growth inhibition assay | Inhibition of human SN12C cell growth in a cell viability assay, IC50 = 13.2299 μM. | SANGER | |||
| HCC1143 | Growth inhibition assay | Inhibition of human HCC1143 cell growth in a cell viability assay, IC50 = 13.3355 μM. | SANGER | |||
| IST-SL2 | Growth inhibition assay | Inhibition of human IST-SL2 cell growth in a cell viability assay, IC50 = 13.3438 μM. | SANGER | |||
| RPMI-7951 | Growth inhibition assay | Inhibition of human RPMI-7951 cell growth in a cell viability assay, IC50 = 13.455 μM. | SANGER | |||
| NCI-H441 | Growth inhibition assay | Inhibition of human NCI-H441 cell growth in a cell viability assay, IC50 = 13.5565 μM. | SANGER | |||
| COLO-320-HSR | Growth inhibition assay | Inhibition of human COLO-320-HSR cell growth in a cell viability assay, IC50 = 13.5724 μM. | SANGER | |||
| SW756 | Growth inhibition assay | Inhibition of human SW756 cell growth in a cell viability assay, IC50 = 13.621 μM. | SANGER | |||
| KP-4 | Growth inhibition assay | Inhibition of human KP-4 cell growth in a cell viability assay, IC50 = 13.6226 μM. | SANGER | |||
| MMAC-SF | Growth inhibition assay | Inhibition of human MMAC-SF cell growth in a cell viability assay, IC50 = 13.7027 μM. | SANGER | |||
| COR-L23 | Growth inhibition assay | Inhibition of human COR-L23 cell growth in a cell viability assay, IC50 = 13.7822 μM. | SANGER | |||
| T-24 | Growth inhibition assay | Inhibition of human T-24 cell growth in a cell viability assay, IC50 = 13.9157 μM. | SANGER | |||
| LAN-6 | Growth inhibition assay | Inhibition of human LAN-6 cell growth in a cell viability assay, IC50 = 14.0704 μM. | SANGER | |||
| MDA-MB-415 | Growth inhibition assay | Inhibition of human MDA-MB-415 cell growth in a cell viability assay, IC50 = 14.2034 μM. | SANGER | |||
| HCC1954 | Growth inhibition assay | Inhibition of human HCC1954 cell growth in a cell viability assay, IC50 = 14.3357 μM. | SANGER | |||
| DBTRG-05MG | Growth inhibition assay | Inhibition of human DBTRG-05MG cell growth in a cell viability assay, IC50 = 14.4419 μM. | SANGER | |||
| NCI-H1734 | Growth inhibition assay | Inhibition of human NCI-H1734 cell growth in a cell viability assay, IC50 = 14.5239 μM. | SANGER | |||
| ME-180 | Growth inhibition assay | Inhibition of human ME-180 cell growth in a cell viability assay, IC50 = 14.5317 μM. | SANGER | |||
| IST-MES1 | Growth inhibition assay | Inhibition of human IST-MES1 cell growth in a cell viability assay, IC50 = 14.5903 μM. | SANGER | |||
| MDA-MB-157 | Growth inhibition assay | Inhibition of human MDA-MB-157 cell growth in a cell viability assay, IC50 = 14.6719 μM. | SANGER | |||
| CPC-N | Growth inhibition assay | Inhibition of human CPC-N cell growth in a cell viability assay, IC50 = 14.7602 μM. | SANGER | |||
| HCC1395 | Growth inhibition assay | Inhibition of human HCC1395 cell growth in a cell viability assay, IC50 = 14.9235 μM. | SANGER | |||
| OS-RC-2 | Growth inhibition assay | Inhibition of human OS-RC-2 cell growth in a cell viability assay, IC50 = 15.0178 μM. | SANGER | |||
| A673 | Growth inhibition assay | Inhibition of human A673 cell growth in a cell viability assay, IC50 = 15.3467 μM. | SANGER | |||
| SK-PN-DW | Growth inhibition assay | Inhibition of human SK-PN-DW cell growth in a cell viability assay, IC50 = 15.5224 μM. | SANGER | |||
| K052 | Growth inhibition assay | Inhibition of human K052 cell growth in a cell viability assay, IC50 = 15.8875 μM. | SANGER | |||
| MDA-MB-468 | Growth inhibition assay | Inhibition of human MDA-MB-468 cell growth in a cell viability assay, IC50 = 16.064 μM. | SANGER | |||
| HuP-T3 | Growth inhibition assay | Inhibition of human HuP-T3 cell growth in a cell viability assay, IC50 = 16.2005 μM. | SANGER | |||
| IMR-5 | Growth inhibition assay | Inhibition of human IMR-5 cell growth in a cell viability assay, IC50 = 16.5378 μM. | SANGER | |||
| NCI-H2347 | Growth inhibition assay | Inhibition of human NCI-H2347 cell growth in a cell viability assay, IC50 = 16.8746 μM. | SANGER | |||
| MPP-89 | Growth inhibition assay | Inhibition of human MPP-89 cell growth in a cell viability assay, IC50 = 17.3268 μM. | SANGER | |||
| SBC-1 | Growth inhibition assay | Inhibition of human SBC-1 cell growth in a cell viability assay, IC50 = 17.5739 μM. | SANGER | |||
| CAS-1 | Growth inhibition assay | Inhibition of human CAS-1 cell growth in a cell viability assay, IC50 = 17.6011 μM. | SANGER | |||
| GOTO | Growth inhibition assay | Inhibition of human GOTO cell growth in a cell viability assay, IC50 = 17.6541 μM. | SANGER | |||
| NCI-H1755 | Growth inhibition assay | Inhibition of human NCI-H1755 cell growth in a cell viability assay, IC50 = 17.9131 μM. | SANGER | |||
| TE-9 | Growth inhibition assay | Inhibition of human TE-9 cell growth in a cell viability assay, IC50 = 18.0951 μM. | SANGER | |||
| PC-14 | Growth inhibition assay | Inhibition of human PC-14 cell growth in a cell viability assay, IC50 = 18.3904 μM. | SANGER | |||
| NCI-H1792 | Growth inhibition assay | Inhibition of human NCI-H1792 cell growth in a cell viability assay, IC50 = 18.4986 μM. | SANGER | |||
| NCI-H1650 | Growth inhibition assay | Inhibition of human NCI-H1650 cell growth in a cell viability assay, IC50 = 18.5279 μM. | SANGER | |||
| HSC-4 | Growth inhibition assay | Inhibition of human HSC-4 cell growth in a cell viability assay, IC50 = 18.5343 μM. | SANGER | |||
| CAMA-1 | Growth inhibition assay | Inhibition of human CAMA-1 cell growth in a cell viability assay, IC50 = 18.6634 μM. | SANGER | |||
| UACC-257 | Growth inhibition assay | Inhibition of human UACC-257 cell growth in a cell viability assay, IC50 = 19.9329 μM. | SANGER | |||
| TGBC24TKB | Growth inhibition assay | Inhibition of human TGBC24TKB cell growth in a cell viability assay, IC50 = 20.0008 μM. | SANGER | |||
| DV-90 | Growth inhibition assay | Inhibition of human DV-90 cell growth in a cell viability assay, IC50 = 20.1541 μM. | SANGER | |||
| NCI-H1563 | Growth inhibition assay | Inhibition of human NCI-H1563 cell growth in a cell viability assay, IC50 = 20.355 μM. | SANGER | |||
| NCI-H1703 | Growth inhibition assay | Inhibition of human NCI-H1703 cell growth in a cell viability assay, IC50 = 20.4318 μM. | SANGER | |||
| EVSA-T | Growth inhibition assay | Inhibition of human EVSA-T cell growth in a cell viability assay, IC50 = 20.6831 μM. | SANGER | |||
| NCI-H1651 | Growth inhibition assay | Inhibition of human NCI-H1651 cell growth in a cell viability assay, IC50 = 20.8933 μM. | SANGER | |||
| SW982 | Growth inhibition assay | Inhibition of human SW982 cell growth in a cell viability assay, IC50 = 20.9701 μM. | SANGER | |||
| EKVX | Growth inhibition assay | Inhibition of human EKVX cell growth in a cell viability assay, IC50 = 20.9978 μM. | SANGER | |||
| WSU-NHL | Growth inhibition assay | Inhibition of human WSU-NHL cell growth in a cell viability assay, IC50 = 21.1658 μM. | SANGER | |||
| Ramos-2G6-4C10 | Growth inhibition assay | Inhibition of human Ramos-2G6-4C10 cell growth in a cell viability assay, IC50 = 21.5523 μM. | SANGER | |||
| RVH-421 | Growth inhibition assay | Inhibition of human RVH-421 cell growth in a cell viability assay, IC50 = 21.7603 μM. | SANGER | |||
| CAPAN-1 | Growth inhibition assay | Inhibition of human CAPAN-1 cell growth in a cell viability assay, IC50 = 21.9322 μM. | SANGER | |||
| KOSC-2 | Growth inhibition assay | Inhibition of human KOSC-2 cell growth in a cell viability assay, IC50 = 22.3163 μM. | SANGER | |||
| U-2-OS | Growth inhibition assay | Inhibition of human U-2-OS cell growth in a cell viability assay, IC50 = 22.5771 μM. | SANGER | |||
| MS-1 | Growth inhibition assay | Inhibition of human MS-1 cell growth in a cell viability assay, IC50 = 22.8045 μM. | SANGER | |||
| SJSA-1 | Growth inhibition assay | Inhibition of human SJSA-1 cell growth in a cell viability assay, IC50 = 22.8269 μM. | SANGER | |||
| LB1047-RCC | Growth inhibition assay | Inhibition of human LB1047-RCC cell growth in a cell viability assay, IC50 = 23.2169 μM. | SANGER | |||
| RMG-I | Growth inhibition assay | Inhibition of human RMG-I cell growth in a cell viability assay, IC50 = 23.2567 μM. | SANGER | |||
| FTC-133 | Growth inhibition assay | Inhibition of human FTC-133 cell growth in a cell viability assay, IC50 = 23.3495 μM. | SANGER | |||
| L-363 | Growth inhibition assay | Inhibition of human L-363 cell growth in a cell viability assay, IC50 = 23.459 μM. | SANGER | |||
| CAL-120 | Growth inhibition assay | Inhibition of human CAL-120 cell growth in a cell viability assay, IC50 = 23.465 μM. | SANGER | |||
| ECC4 | Growth inhibition assay | Inhibition of human ECC4 cell growth in a cell viability assay, IC50 = 24.3197 μM. | SANGER | |||
| ESS-1 | Growth inhibition assay | Inhibition of human ESS-1 cell growth in a cell viability assay, IC50 = 24.5306 μM. | SANGER | |||
| OAW-28 | Growth inhibition assay | Inhibition of human OAW-28 cell growth in a cell viability assay, IC50 = 24.5363 μM. | SANGER | |||
| BT-20 | Growth inhibition assay | Inhibition of human BT-20 cell growth in a cell viability assay, IC50 = 24.7808 μM. | SANGER | |||
| MLMA | Growth inhibition assay | Inhibition of human MLMA cell growth in a cell viability assay, IC50 = 25.2094 μM. | SANGER | |||
| DMS-114 | Growth inhibition assay | Inhibition of human DMS-114 cell growth in a cell viability assay, IC50 = 25.6084 μM. | SANGER | |||
| NCI-H209 | Growth inhibition assay | Inhibition of human NCI-H209 cell growth in a cell viability assay, IC50 = 26.8698 μM. | SANGER | |||
| no-10 | Growth inhibition assay | Inhibition of human no-10 cell growth in a cell viability assay, IC50 = 27.1232 μM. | SANGER | |||
| Saos-2 | Growth inhibition assay | Inhibition of human Saos-2 cell growth in a cell viability assay, IC50 = 27.301 μM. | SANGER | |||
| AM-38 | Growth inhibition assay | Inhibition of human AM-38 cell growth in a cell viability assay, IC50 = 27.3468 μM. | SANGER | |||
| SiHa | Growth inhibition assay | Inhibition of human SiHa cell growth in a cell viability assay, IC50 = 27.4329 μM. | SANGER | |||
| SK-MM-2 | Growth inhibition assay | Inhibition of human SK-MM-2 cell growth in a cell viability assay, IC50 = 27.4479 μM. | SANGER | |||
| NCI-H2126 | Growth inhibition assay | Inhibition of human NCI-H2126 cell growth in a cell viability assay, IC50 = 27.5533 μM. | SANGER | |||
| EHEB | Growth inhibition assay | Inhibition of human EHEB cell growth in a cell viability assay, IC50 = 27.6218 μM. | SANGER | |||
| CCF-STTG1 | Growth inhibition assay | Inhibition of human CCF-STTG1 cell growth in a cell viability assay, IC50 = 27.8125 μM. | SANGER | |||
| CaR-1 | Growth inhibition assay | Inhibition of human CaR-1 cell growth in a cell viability assay, IC50 = 28.2491 μM. | SANGER | |||
| C8166 | Growth inhibition assay | Inhibition of human C8166 cell growth in a cell viability assay, IC50 = 28.3758 μM. | SANGER | |||
| LC-1F | Growth inhibition assay | Inhibition of human LC-1F cell growth in a cell viability assay, IC50 = 29.4042 μM. | SANGER | |||
| DJM-1 | Growth inhibition assay | Inhibition of human DJM-1 cell growth in a cell viability assay, IC50 = 29.5518 μM. | SANGER | |||
| SIMA | Growth inhibition assay | Inhibition of human SIMA cell growth in a cell viability assay, IC50 = 29.6439 μM. | SANGER | |||
| SAS | Growth inhibition assay | Inhibition of human SAS cell growth in a cell viability assay, IC50 = 30.4639 μM. | SANGER | |||
| EW-3 | Growth inhibition assay | Inhibition of human EW-3 cell growth in a cell viability assay, IC50 = 30.9582 μM. | SANGER | |||
| NCI-H28 | Growth inhibition assay | Inhibition of human NCI-H28 cell growth in a cell viability assay, IC50 = 31.3755 μM. | SANGER | |||
| EM-2 | Growth inhibition assay | Inhibition of human EM-2 cell growth in a cell viability assay, IC50 = 31.719 μM. | SANGER | |||
| NCI-H1693 | Growth inhibition assay | Inhibition of human NCI-H1693 cell growth in a cell viability assay, IC50 = 31.976 μM. | SANGER | |||
| D-283MED | Growth inhibition assay | Inhibition of human D-283MED cell growth in a cell viability assay, IC50 = 32.0517 μM. | SANGER | |||
| U-118-MG | Growth inhibition assay | Inhibition of human U-118-MG cell growth in a cell viability assay, IC50 = 32.5513 μM. | SANGER | |||
| SW1417 | Growth inhibition assay | Inhibition of human SW1417 cell growth in a cell viability assay, IC50 = 34.7105 μM. | SANGER | |||
| CP50-MEL-B | Growth inhibition assay | Inhibition of human CP50-MEL-B cell growth in a cell viability assay, IC50 = 34.857 μM. | SANGER | |||
| RO82-W-1 | Growth inhibition assay | Inhibition of human RO82-W-1 cell growth in a cell viability assay, IC50 = 35.4145 μM. | SANGER | |||
| BOKU | Growth inhibition assay | Inhibition of human BOKU cell growth in a cell viability assay, IC50 = 35.5508 μM. | SANGER | |||
| NCI-H1623 | Growth inhibition assay | Inhibition of human NCI-H1623 cell growth in a cell viability assay, IC50 = 36.3726 μM. | SANGER | |||
| KP-N-YS | Growth inhibition assay | Inhibition of human KP-N-YS cell growth in a cell viability assay, IC50 = 36.9577 μM. | SANGER | |||
| KINGS-1 | Growth inhibition assay | Inhibition of human KINGS-1 cell growth in a cell viability assay, IC50 = 37.34 μM. | SANGER | |||
| NCI-H64 | Growth inhibition assay | Inhibition of human NCI-H64 cell growth in a cell viability assay, IC50 = 38.2943 μM. | SANGER | |||
| UACC-62 | Growth inhibition assay | Inhibition of human UACC-62 cell growth in a cell viability assay, IC50 = 38.3702 μM. | SANGER | |||
| PFSK-1 | Growth inhibition assay | Inhibition of human PFSK-1 cell growth in a cell viability assay, IC50 = 38.3732 μM. | SANGER | |||
| EW-11 | Growth inhibition assay | Inhibition of human EW-11 cell growth in a cell viability assay, IC50 = 38.8191 μM. | SANGER | |||
| Hs-578-T | Growth inhibition assay | Inhibition of human Hs-578-T cell growth in a cell viability assay, IC50 = 39.0678 μM. | SANGER | |||
| RXF393 | Growth inhibition assay | Inhibition of human RXF393 cell growth in a cell viability assay, IC50 = 43.0187 μM. | SANGER | |||
| LU-65 | Growth inhibition assay | Inhibition of human LU-65 cell growth in a cell viability assay, IC50 = 43.3721 μM. | SANGER | |||
| UACC-893 | Growth inhibition assay | Inhibition of human UACC-893 cell growth in a cell viability assay, IC50 = 43.8964 μM. | SANGER | |||
| IST-SL1 | Growth inhibition assay | Inhibition of human IST-SL1 cell growth in a cell viability assay, IC50 = 44.4804 μM. | SANGER | |||
| NCI-H1581 | Growth inhibition assay | Inhibition of human NCI-H1581 cell growth in a cell viability assay, IC50 = 44.9621 μM. | SANGER | |||
| U-266 | Growth inhibition assay | Inhibition of human U-266 cell growth in a cell viability assay, IC50 = 44.9791 μM. | SANGER | |||
| GI-ME-N | Growth inhibition assay | Inhibition of human GI-ME-N cell growth in a cell viability assay, IC50 = 45.1745 μM. | SANGER | |||
| CHP-126 | Growth inhibition assay | Inhibition of human CHP-126 cell growth in a cell viability assay, IC50 = 45.5137 μM. | SANGER | |||
| K5 | Growth inhibition assay | Inhibition of human K5 cell growth in a cell viability assay, IC50 = 46.2963 μM. | SANGER | |||
| GCT | Growth inhibition assay | Inhibition of human GCT cell growth in a cell viability assay, IC50 = 46.7832 μM. | SANGER | |||
| T47D | Growth inhibition assay | Inhibition of human T47D cell growth in a cell viability assay, IC50 = 46.8029 μM. | SANGER | |||
| TGBC1TKB | Growth inhibition assay | Inhibition of human TGBC1TKB cell growth in a cell viability assay, IC50 = 46.968 μM. | SANGER | |||
| ES5 | Growth inhibition assay | Inhibition of human ES5 cell growth in a cell viability assay, IC50 = 47.748 μM. | SANGER | |||
| COR-L88 | Growth inhibition assay | Inhibition of human COR-L88 cell growth in a cell viability assay, IC50 = 47.8867 μM. | SANGER | |||
| LS-513 | Growth inhibition assay | Inhibition of human LS-513 cell growth in a cell viability assay, IC50 = 48.4939 μM. | SANGER | |||
| NB69 | Growth inhibition assay | Inhibition of human NB69 cell growth in a cell viability assay, IC50 = 49.074 μM. | SANGER | |||
| UMC-11 | Growth inhibition assay | Inhibition of human UMC-11 cell growth in a cell viability assay, IC50 = 49.6957 μM. | SANGER | |||
| Click to View More Cell Line Experimental Data | ||||||
Mechanism of Action
| Description | Tipifarnib is a potent and specific farnesyltransferase (FTase) inhibitor with IC50 of 0.6 nM, its anti-proliferative effects are most prominent in H-ras or N-ras mutant cells. Phase 3. | ||
|---|---|---|---|
| Features | A potent and selective farnesyl protein transferase inhibitor with significant antitumor effects. | ||
| Targets |
|
In vitro |
||||
| In vitro | Using Tipifarnib 5 μM for 72 hours, the percentage of apoptotic cells is significantly higher in drug-treated compared to DMSO-treated LGL T-cells. Using T-cells from healthy donors, Tipifarnib reduces the percentage of IFNγ-positive cells in a time-dependent manner. Tipifarnib reduces the amount of activated Ras in precipitates compared to DMSO. [2] Tipifarnib exerts selective in vitro toxicity against clonal MDS hematopoiesis at concentrations less than 10 nM the effect being more prominent in white cell progenitors. This action is not due to apoptosis induction as both normal and MDS progenitors displays equivalent DiOC3 and annexin V expression up to 72 hours after exposure to Tipifarnib. [3] Combining Tipifarnib with 10 nM 4-OH-ICI 46474 in the presence of E2 reduces the IC50 8-fold from 400 to 50 nM. [4] Tipifarnib induces apoptosis in U937 cells. [5] In addition, Tipifarnib inhibits isolated human farnesyltransferase for a lamin B peptide and for the K-RasB peptide with IC50 of 0.86 nM and 7.9 nM, respectively. [6] |
|||
|---|---|---|---|---|
| Cell Research | Cell lines | MACS-selected CD34+ cells | ||
| Concentrations | 2.5 nM, 10 nM, 25 nM and 50 nM | |||
| Incubation Time | 48 hours | |||
| Method | MACS-selected CD34+ cells are seeded in Methocult 4435 'complete' 1% bovine serum albumin, 3 U/mL recombinant human (rh) erythropoietin, 0.1 mM 2-mercaptoethanol, 2 mM L-glutamine and the following cytokines: 50 ng/mL rh stem cell factor, 20 ng/mL rh GM-CSF, 20 ng/mL rh IL-3, 20 ng/mL rh IL-6 and 20 ng/mL h G-CSF. DMSO or Tipifarnib is added at the concentrations of 2.5, 10, 25 and 50 nM at day 1. All cultures are performed in duplicates and the numbers of colonies are scored after 14 days of incubation at 37 °C in a humidified incubator containing 5% CO2 |
|||
| Experimental Result Images | Methods | Biomarkers | Images | PMID |
| Western blot | Caspase-12 / PARP1 Rheb / p-mTOR / mTOR / p-P70S6K / P70 S6K / p-S6 / S6 Bcl-2 / Bcl-xl / Mcl-1 / Bax / Bak / Puma / Noxa / Bim / Lamin B HDJ-2 / N-Ras / FKBP51 / p-AKT / AKT p-c-Raf / c-Raf / p-MEK / MEK / p-ERK / ERK |
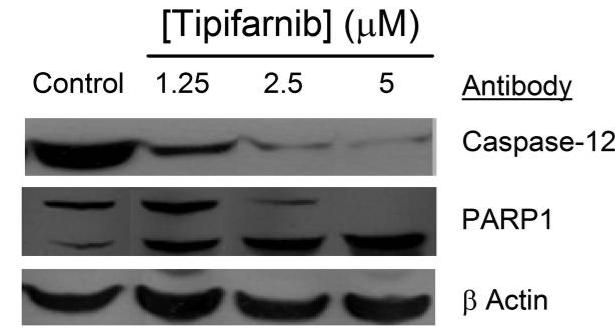
|
21378206 | |
| Growth inhibition assay | Cell viability |
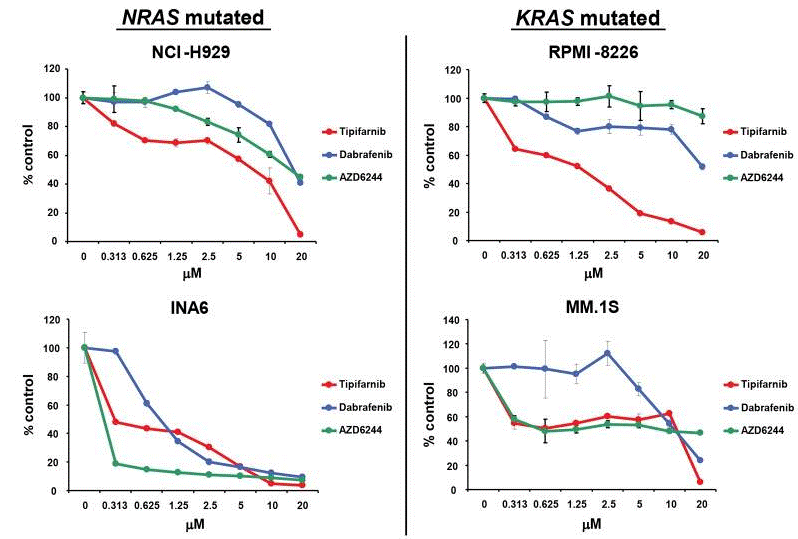
|
26630652 | |
In Vivo |
||
| In vivo | Ki-67 is lower in the tumors treated with E2 withdrawal plus R115777 compared with E2 withdrawal alone. The combination of ICI 46474 and R115777 results in significantly lower Ki-67 compared with either ICI 46474 or R115777 alone (mean of 5% versus 16.9% and 67.3%, respectively). [4] In contrast, no significant difference in apoptotic scores is seen between the treatment groups. R115777 alone also reduces the CTI compared with control. The combination of ICI 46474 and R115777 or R115777 coupled with E2 withdrawal is most effective at lowering the CTI (0.8 and 0.7, respectively), which may account for the decrease in tumor volume. [4] |
|
|---|---|---|
| Animal Research | Animal Models | Female ovariectomized Ncr foxhead nude mice |
| Dosages | 50 mg/kg | |
| Administration | Oral gavage | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05693090 | Withdrawn | NSCLC |
Kura Oncology Inc. |
February 1 2023 | Phase 1 |
| NCT04997902 | Recruiting | HNSCC |
Kura Oncology Inc. |
December 7 2021 | Phase 1|Phase 2 |
| NCT04865159 | Terminated | Advanced Solid Tumor |
Kura Oncology Inc. |
May 6 2021 | Phase 1 |
| NCT03719690 | Active not recruiting | HRAS Gene Mutation|HNSCC |
Kura Oncology Inc. |
November 5 2018 | Phase 2 |
| NCT02807272 | Completed | Leukemia Myelomonocytic Chronic |
Kura Oncology Inc. |
October 2016 | Phase 2 |
References |
|
Chemical Information
| Molecular Weight | 489.4 | Formula | C27H22Cl2N4O |
| CAS No. | 192185-72-1 | SDF | Download Tipifarnib SDF |
| Synonyms | R115777, IND 58359 | ||
| Smiles | CN1C=NC=C1C(C2=CC=C(C=C2)Cl)(C3=CC4=C(C=C3)N(C(=O)C=C4C5=CC(=CC=C5)Cl)C)N | ||
Storage and Stability
| Storage (From the date of receipt) | |||
|
In vitro |
DMSO : 14 mg/mL ( (28.6 mM) Moisture-absorbing DMSO reduces solubility. Please use fresh DMSO.) Ethanol : 9 mg/mL Water : Insoluble |
Molecular Weight Calculator |
|
In vivo Add solvents to the product individually and in order. |
In vivo Formulation Calculator |
|||||
Preparing Stock Solutions
Molarity Calculator
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Tech Support
Answers to questions you may have can be found in the inhibitor handling instructions. Topics include how to prepare stock solutions, how to store inhibitors, and issues that need special attention for cell-based assays and animal experiments.
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
* Indicates a Required Field





































