- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Thioguanine DNA Methyltransferase inhibitor
Cat.No.S1774
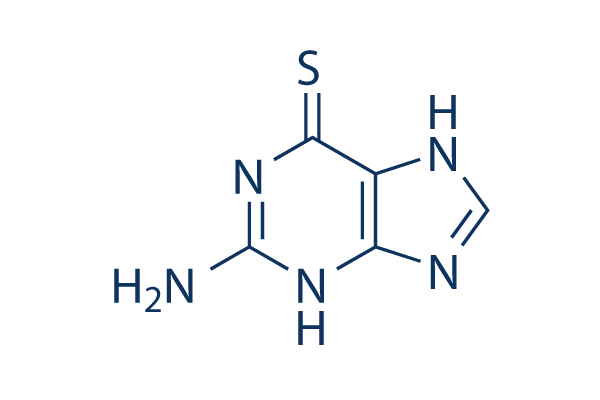
Chemical Structure
Molecular Weight: 167.19
Jump to
Quality Control
Batch:
Purity:
99.93%
99.93
| Related Targets | HDAC JAK BET Histone Methyltransferase PKC PARP HIF PRMT EZH2 AMPK |
|---|---|
| Other DNA Methyltransferase Products | RG108 SGI-1027 Zebularine (NSC 309132) Gamma-Oryzanol CM272 GSK3685032 β-thujaplicin GSK-3484862 SF3B1 Antibody [G23D1] DNMT3A Antibody [E19E6] |
Solubility
|
In vitro |
DMSO
: 17 mg/mL
(101.68 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 167.19 | Formula | C5H5N5S |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 154-42-7 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | NSC 752, 6-Thioguanine, 2-Amino-6-purinethiol | Smiles | C1=NC2=C(N1)C(=S)N=C(N2)N | ||
Mechanism of Action
| Targets/IC50/Ki |
DNMT1
|
|---|---|
| In vitro |
Thioguanine incorporation alters the DNA cleavage induced by topoisomerase II in the presence and absence of etoposide. This compound alters the structure and lowers the thermal stability of duplex DNA, but duplex DNA can be formed in the presence of 6SG. Its induced apopotosis is similarly observed in both mismatch repair-proficient and -deficient HCT116 and HeLa cells. This chemical integrates into DNA and unlike the canonical DNA bases, it is a strong UVA chromophore with an absorbance maximum at 342 nm. It is a photosensitizer and a source of reactive oxygen species. In canine lymphoma cells, this compound significantly decreases DNMT1 protein and global DNA methylation. |
| In vivo |
Thioguanine is as efficient as a PARP inhibitor in selectively killing BRCA2-defective tumors in a xenograft model. This compound efficiently kills such BRCA1-defective PARP inhibitor-resistant tumors. It could kill cells and tumors that have gained resistance to PARP inhibitors or cisplatin through genetic reversion of the BRCA2 gene. |
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT03022747 | Unknown status | Lymphoblastic Leukemia Acute Childhood |
Vastra Gotaland Region |
January 2017 | Phase 2 |
| NCT04304950 | Completed | Inflammatory Bowel Diseases |
Rush University Medical Center |
April 25 2016 | Phase 4 |
| NCT00548431 | Completed | Leukemia Lymphocytic Acute |
Rigshospitalet Denmark |
December 2007 | Phase 2 |
| NCT00098111 | Terminated | Crohn''s Disease |
Massachusetts General Hospital |
April 2005 | Phase 3 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































