research use only
Raltegravir Integrase inhibitor
Cat.No.S2005
Chemical Structure
Molecular Weight: 444.42
Quality Control
| Related Targets | Bacterial Antibiotics Anti-infection Fungal Antiviral COVID-19 Parasite Reverse Transcriptase HIV HCV Protease |
|---|---|
| Other Integrase Inhibitors | MK-2048 BMS-707035 Robinetin Lavendustin B |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| MT4 | Antiviral assay | Antiviral activity against Human immunodeficiency virus 1 3B infected in human MT4 cells assessed as inhibition of viral replication, EC50 = 0.0014 μM. | 18541726 | |||
| MT-4 | Antiviral assay | Antiviral activity against Human immunodeficiency virus 1 3B infected in human MT-4 cells assessed as inhibition of viral replication, EC50 = 0.0018 μM. | 18160521 | |||
| MT-4 | Antiviral assay | Antiviral activity against vesicular stomatitis virus G-pseudotyped Human immunodeficiency virus infected in human MT-4 cells assessed as inhibition of viral replication, EC50 = 0.002 μM. | 18160521 | |||
| MT2 | Antiviral assay | 3 to 4 days | Antiviral activity against HIV1 NL4-3 infected in human MT2 cells measured after 3 to 4 days by luciferase reporter gene assay, EC50 = 0.002 μM. | 32081010 | ||
| MT-2 | Antiviral assay | Antiviral activity against HIV1 3B infected in human MT-2 cells by two fold dilution method in presence of 10% FBS, EC50 = 0.003 μM. | 19104010 | |||
| MT2 | Antiviral assay | 5 days | Antiviral activity against HIV1 3B infected in human MT2 cells after 5 days by chemiluminescence in presence of fetal bovine serum, EC50 = 0.004 μM. | 19285389 | ||
| HOS | Antiviral assay | Antiviral activity against HIV1 harboring wild-type integrase infected in human HOS cells, EC50 = 0.004 μM. | 21493066 | |||
| HOS | Antiviral assay | 3 hrs | Antiviral activity against wild-type Human immunodeficiency virus 1 infected in HOS cells preincubated for 3 hrs followed by viral infection measured after 48 hrs by luciferase reporter gene assay, EC50 = 0.004 μM. | 24471816 | ||
| MT2 | Antiviral assay | 5 days | Antiviral activity against HIV1 3B in MT2 cells after 5 days, EC50 = 0.006 μM. | 18207398 | ||
| MT-4 | Antiviral assay | Antiviral activity against HIV 1 RIN harboring integrase gene infected in MT-4 cells assessed as inhibition of virus-induced cytopathic effect by MTT assay, EC50 = 0.006 μM. | 18378713 | |||
| MT4 | Antiviral assay | Antiviral activity against HIV1 3B infected in human MT4 cells assessed as inhibition of virus-induced cytopathic effect by MTT assay, EC50 = 0.006 μM. | 24900718 | |||
| MT4 | Antiviral assay | Antiviral activity against HIV-1 3B infected in human MT4 cells assessed as protection against virus-induced cytopathic effect by MTT assay, EC50 = 0.006 μM. | 24793360 | |||
| MT4 | Antiviral assay | Antiviral activity against Human immunodeficiency virus 1 3B infected in human MT4 cells assessed as inhibition of virus induced cytopathic effect, EC50 = 0.0061 μM. | 20479206 | |||
| MT4 | Function assay | 1 hr | Inhibition of wild type HIV1 3B infected in human MT4 cells using cells pre-incubated with compound for 1 hr measured 4 days post viral infection by MTT assay, IC50 = 0.0062 μM. | 22963135 | ||
| MT4 | Antiviral assay | Antiviral activity against HIV1 3B infected in human MT4 cells assessed as reduction of virus-induced cytopathogenicity by MTT assay, EC50 = 0.0064 μM. | 19447621 | |||
| MT-4 | Antiviral assay | Antiviral activity against HIV 1 NL4.3 harboring integrase L74M mutant infected in human MT-4 cells assessed as inhibition of virus-induced cytopathic effect by MTT assay, EC50 = 0.007 μM. | 18378713 | |||
| MT4 | Antiviral assay | Antiviral activity against HIV1 3B infected in human MT4 cells assessed as inhibition of viral-induced cytopathic effect by MTT assay, EC50 = 0.0078 μM. | 28951095 | |||
| MT-4 | Antiviral assay | Antiviral activity against HIV1 3B infected in human MT-4 cells by two fold dilution method in presence of 10% FBS, EC50 = 0.008 μM. | 19104010 | |||
| MT-4 | Antiviral assay | Antiviral activity against HIV 1 NL4.3 integrase S230N mutant infected in human MT-4 cells assessed as inhibition of virus-induced cytopathic effect by MTT assay, EC50 = 0.009 μM. | 18378713 | |||
| MT4 | Antiviral assay | 48 to 72 hrs | Antiviral activity against HIV1 infected in human MT4 cells expressing GFP incubated for 48 to 72 hrs in absence of 50% normal human serum by multiple round viral replication kinetics assay, IP = 0.009 μM. | 26397965 | ||
| FL-4 | Antiviral assay | Antiviral activity against Feline immunodeficiency virus infected in cat FL-4 cells assessed as viral RNA production by RT-qPCR, EC50 = 0.00998 μM. | 24813732 | |||
| MT-4 | Antiviral assay | Antiviral activity against HIV 1 RIN harboring integrase gene infected in MT-4 cells assessed as inhibition of virus-induced cytopathic effect by MTT assay after 20 passages selected in presence of compound, EC50 = 0.01 μM. | 18378713 | |||
| HeLa | Antiviral assay | 48 hrs | Antiviral activity against VSV-G pseudotyped HIV1 lentiviral particles infected in human HeLa cells incubated for 48 hrs by spectrofluorometry, IC50 = 0.01 μM. | 22858300 | ||
| cat FL-4 | Antiviral assay | 7 days | Antiviral activity against Feline immunodeficiency virus infected in cat FL-4 cells assessed as inhibition of viral replication after 7 days by quantitative RT-PCR analysis, EC50 = 0.01 μM. | 25702849 | ||
| cat FL-4 | Antiviral assay | 7 days | Antiviral activity against FIV infected in cat FL-4 cells assessed as reduction in viral RNA level measured after 7 days by qRT-PCR analysis, EC50 = 0.01 μM. | 31395510 | ||
| MT4 | Antiviral assay | Antiviral activity against HIV2 ROD 3B infected in human MT4 cells assessed as inhibition of viral-induced cytopathic effect by MTT assay, EC50 = 0.011 μM. | 28951095 | |||
| MT4 | Antiviral assay | Antiviral activity against Human immunodeficiency virus 1 3B infected in MT4 cells assessed as protection against virus-induced cytopathic effect measured 5 days post infection by MTT assay, EC50 = 0.0117 μM. | 29940462 | |||
| MT4 | Antiviral assay | Antiviral activity against HIV1 3B assessed as inhibition of viral-induced cytopathic effect in human MT4 cells, EC50 = 0.013 μM. | 18417342 | |||
| MT4 | Antiviral assay | Antiviral activity against HIV1 infected in human MT4 cells assessed as inhibition of virus-induced cytopathic effect, EC50 = 0.013 μM. | 20630765 | |||
| MT4 | Antiviral assay | Antiviral activity against HIV1 3B infected in human MT4 cells assessed as inhibition of virus-induced cytopathic effect by MTT assay, EC50 = 0.013 μM. | 21227550 | |||
| BL21(DE3) | Function assay | 1 hr | Inhibition of recombinant HIV-1 integrase stand transfer activity expressed in Escherichia coli BL21(DE3) cells using 32P-labeled DNA substrate after 1 hr by densitometric analysis, IC50 = 0.013 μM. | 26451771 | ||
| MT-4 | Antiviral assay | Antiviral activity against HIV 1 NL4.3 integrase E92Q mutant infected in human MT-4 cells assessed as inhibition of virus-induced cytopathic effect by MTT assay, EC50 = 0.015 μM. | 18378713 | |||
| MT4 | Antiviral assay | 5 days | Antiviral activity against HIV1 3B infected in human MT4 cells incubated for 5 days by MTT assay, EC50 = 0.015 μM. | 31324562 | ||
| MT2 | Antiviral assay | Antiviral activity against HIV in MT2 cells in presence of 35 mg/mL human serum albumin, EC50 = 0.016 μM. | 18207398 | |||
| MT-4 | Antiviral assay | 5 days | Antiviral activity against HIV1 3B/LAI infected in human MT-4 cells assessed as inhibition of virus-induced cytopathic effect measured survival after 5 days by MTT assay, EC50 = 0.016 μM. | 24124919 | ||
| HeLa-CD4-LTR-beta-gal | Antiviral assay | 1 day | Antiviral activity against HIV-1 3B infected in human HeLa-CD4-LTR-beta-gal cells after 1 day by spectroscopic analysis, EC50 = 0.016 μM. | 25629256 | ||
| MT-2 | Antiviral assay | Antiviral activity against HIV1 3B infected in human MT-2 cells by two fold dilution method in presence of 10% FBS, HSA and alpha1-AGP, EC50 = 0.018 μM. | 19104010 | |||
| MT4 | Antiviral assay | Antiviral activity against HIV1 3B in human MT4 cells in presence of 10% heat-activated fetal bovine serum, IC95 = 0.019 μM. | 18763751 | |||
| MT2 | Antiviral assay | 5 days | Antiviral activity against HIV1 3B infected in human MT2 cells after 5 days by chemiluminescence in presence of human serum albumin, EC50 = 0.02 μM. | 19285389 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against HIV2 ROD infected in human MT4 cells incubated for 5 days by MTT assay, EC50 = 0.02 μM. | 31324562 | ||
| TZM-b1 | Antiviral assay | 1 hr | Antiviral activity against HIV-1 NL4.3 infected in human TZM-b1 cells pretreated for 1 hr followed by viral infection measured after 2 days by CPRG assay, IC50 = 0.021 μM. | 28408224 | ||
| CEM-SS | Antiviral assay | 6 days | Antiviral activity against HIV1 3B infected in human CEM-SS cells assessed as reduction of virus-induced cytopathic effect after 6 days by MTS assay, EC50 = 0.023 μM. | 27283261 | ||
| HeLa | Antiviral assay | 3 days | Antiviral activity against HIV1 3B infected in human HeLa cells expressing CD4-LTR-beta-gal assessed as inhibition of viral replication after 3 days by reporter gene assay, EC50 = 0.0236 μM. | 24684270 | ||
| HeLa-CD4-LTR-beta-gal | Antiviral assay | Antiviral activity against HIV-1 3B infected in human HeLa-CD4-LTR-beta-gal cells assessed as inhibition of viral replication by SpectraMax GEMINI-XS plate reader analysis, EC50 = 0.0236 μM. | 25961960 | |||
| HeLa | Antiviral assay | 2 days | Antiviral activity against HIV1 3B infected in human HeLa cells expressing CD4 assessed as inhibition of viral replication after 2 days by beta-galactosidase reporter gene assay, EC50 = 0.024 μM. | 24124919 | ||
| MT-4 | Antiviral assay | Antiviral activity against HIV 1 RIN HIV 1 RIN harboring integrase gene infected in MT-4 cells assessed as inhibition of virus-induced cytopathic effect by MTT assay after 40 passages selected in presence of compound, EC50 = 0.025 μM. | 18378713 | |||
| TZM-bl | Antiviral assay | 48 hrs | Antiviral activity against Human immunodeficiency virus 1 infected in human TZM-bl cells assessed as inhibition of viral replication after 48 hrs by inverted microscopic analysis, EC50 = 0.025 μM. | 22154762 | ||
| MT4 | Function assay | 1 hr | Inhibition of wild type HIV1 3B infected in human MT4 cells using cells pre-incubated with compound for 1 hr measured 4 days post viral infection in presence of 20 mg/ml HSA by MTT assay, IC50 = 0.029 μM. | 22963135 | ||
| P4R5 MAGI | Antiviral assay | 24 hrs | Antiviral activity against HIV1 infected in CD4/CXCR4/CCR5 expressing human P4R5 MAGI cells preincubated with cells for 24 hrs followed by viral infection measured after 48 hrs by beta-galactosidase reporter gene assay, EC50 = 0.03 μM. | 30031976 | ||
| P4R5 | Antiviral assay | 24 hrs | Antiviral activity against HIV1 infected in CD4/CXCR4/CCR5 expressing human P4R5 cells assessed as inhibition of viral replication preincubated with cells for 24 hrs followed by viral infection measured after 48 hrs by beta-galactosidase reporter gene ass, EC50 = 0.03 μM. | 28525279 | ||
| P4R5 | Antiviral assay | 24 hrs | Antiviral activity against HIV1 infected in human P4R5 cells assessed as reduction in viral infection preincubated with cells for 24 hrs followed by viral infection measured after 48 hrs by fluorescence based beta-galactosidase reporter gene based MAGI as, EC50 = 0.03 μM. | 29031062 | ||
| P4R5 | Antiviral assay | 24 hrs | Antiviral activity against HIV-1 infected in human P4R5 cells assessed as reduction in viral replication preincubated for 24 hrs followed by viral infection and measured after 48 hrs by MAGI assay, EC50 = 0.03 μM. | 30739822 | ||
| P4R5 | Antiviral assay | 24 hrs | Antiviral activity against HIV1 infected in human P4R5 cells assessed as reduction of virus replication preincubated with cells for 24 hrs followed by viral infection for 48 hrs by 4-methylumbelliferylgalactoside-based MAGI assay, EC50 = 0.03 μM. | 27283261 | ||
| MT4 | Antiviral assay | Antiviral activity against HIV1 3B in human MT4 cells in presence of 50% normal human serum, IC95 = 0.031 μM. | 18763751 | |||
| MT4 | Antiviral assay | 48 to 72 hrs | Antiviral activity against HIV1 infected in human MT4 cells expressing GFP incubated for 48 to 72 hrs in presence of 50% normal human serum by multiple round viral replication kinetics assay, IP = 0.053 μM. | 26397965 | ||
| BL21(DE3) | Function assay | 1 hr | Inhibition of LEDGF/p75-dependent full length HIV-1 integrase expressed in Escherichia coli BL21(DE3) cells preincubated for 1 hr further incubated for 90 mins with biotin-labeled donor DNA and target DNA by HTRF assay, IC50 = 0.058 μM. | 31442684 | ||
| MT-4 | Antiviral assay | Antiviral activity against HIV 1 RIN HIV 1 RIN harboring integrase gene infected in MT-4 cells assessed as inhibition of virus-induced cytopathic effect by MTT assay after 60 passages selected in presence of compound, EC50 = 0.068 μM. | 18378713 | |||
| HeLaT4 | Antiviral assay | Antiviral activity against single-round HIV1 NLX.Lux-R harboring inactivating mutations in env, vpr and carries firefly luciferase gene in place of nef assessed as level of 2 mins magnetic nanopartials-medated infection in human HeLaT4 cells treated for 1, EC91 = 0.073 μM. | 21060108 | |||
| HeLa-T4 | Antiviral assay | 48 hrs | Antiviral activity against single-round HIV1 NLX.Lux-R harboring inactivating mutations in env, vpr and carries firefly luciferase gene in place of nef infected in human HeLa-T4 cells assessed as luciferase activity after 48 hrs by exogenous RT assay, EC50 = 0.077 μM. | 21060108 | ||
| TZM-bl | Antiviral assay | 48 hrs | Antiviral activity against HIV-1 infected in human TZM-bl cells assessed as inhibition of viral replication for 48 hrs by bright-Glo luciferase assay, EC50 = 0.15 μM. | 26487913 | ||
| HOS | Antiviral assay | 3 hrs | Antiviral activity against Human immunodeficiency virus 1 harboring integrase N155H mutant infected in HOS cells preincubated for 3 hrs followed by viral infection measured after 48 hrs by luciferase reporter gene assay, EC50 = 0.154 μM. | 24471816 | ||
| TZM-bl | Antiviral assay | Antiviral activity against HIV1 NL4.3 infected in human TZM-bl cells measured upto 24 hrs by bright Glo-luciferase reporter gene assay, IC50 = 0.16 μM. | 31557609 | |||
| HOS | Antiviral assay | 3 hrs | Antiviral activity against Human immunodeficiency virus 1 harboring integrase Y143R mutant infected in HOS cells preincubated for 3 hrs followed by viral infection measured after 48 hrs by luciferase reporter gene assay, EC50 = 0.162 μM. | 24471816 | ||
| TZM-bl | Antiviral assay | 1 hr | Antiviral activity against HIV1 NL4.3 infected in human TZM-bl cells expressing CXCR4-tropic incubated for 1 hr prior to infection measured after 48 hrs by luciferase reporter gene assay, IC50 = 0.5 μM. | 24291042 | ||
| TZM-bl | Antiviral assay | 1 hr | Antiviral activity against HIV1 NL4.3 infected in human TZM-bl cells expressing CXCR4-tropic incubated for 1 hr prior to infection measured after 48 hrs by luciferase reporter gene assay, IC50 = 0.50119 μM. | 24291042 | ||
| HeLa-T4 | Antiviral assay | 48 hrs | Antiviral activity against single-round HIV1 NLX.Lux-R harboring inactivating mutations in env, vpr and carries firefly luciferase gene in place of nef infected in human HeLa-T4 cells assessed as luciferase activity after 48 hrs by exogenous RT assay, EC95 = 0.81 μM. | 21060108 | ||
| HOS | Antiviral assay | 3 hrs | Antiviral activity against Human immunodeficiency virus 1 harboring integrase G140S/Q148H double mutant infected in HOS cells preincubated for 3 hrs followed by viral infection measured after 48 hrs by luciferase reporter gene assay, EC50 = 1.9 μM. | 24471816 | ||
| MT2 | Antiviral assay | 3 to 4 days | Antiviral activity against HIV1 NL4-3 harboring G140S/Q148H mutant infected in human MT2 cells measured after 3 to 4 days by luciferase reporter gene assay, EC50 = 2.42 μM. | 32081010 | ||
| HeLaT4 | Antiviral assay | 24 hrs | Antiviral activity against single-round HIV1 NLX.Lux-R harboring inactivating mutations in env, vpr and carries firefly luciferase gene in place of nef assessed as level of infection using human HeLaT4 cells pretreated for 24 hrs followed by exposed to vi, EC50 = 4.6 μM. | 21060108 | ||
| MT4 | Cytotoxicity assay | 5 days | Cytotoxicity in mock-infected human MT4 cells assessed as reduction in cell viability incubated for 5 days by MTT assay, CC50 = 6.51 μM. | 31324562 | ||
| CHO | Function assay | Inhibition of slow delayed inward rectifying potassium current (Iks) in Chinese Hamster Ovary (CHO) cells expressing hKvLQT1/hminK measured using IonWorks Quattro automated patch clamp platform, IC50 = 25.1189 μM. | 25087753 | |||
| U373-MAGI | Antiviral assay | 50 or 100 nM | 72 hrs | Antiviral activity against VSV-G pseudotyped HIV-1 NL4-3 infected in human U373-MAGI cells assessed as reduction in gag level at 50 or 100 nM measured at 72 hrs post infection by qPCR method | 27117260 | |
| U373-MAGI | Antiviral assay | 50 or 100 nM | 72 hrs | Antiviral activity against VSV-G pseudotyped HIV-1 NL4-3 infected in human U373-MAGI cells assessed as reduction in U5-gag level at 50 or 100 nM measured at 72 hrs post infection by qPCR method | 27117260 | |
| MT2 | Antiviral assay | 1 uM | 30 hrs | Antiviral activity against wild-type HIV1 NL4-3 infected infected in human MT2 cells assessed as reduction in HIV1 integrated DNA at 1 uM and measured after 30 hrs post infection by Alu-LTR PCR protocol based method | 30996780 | |
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 89 mg/mL
(200.26 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 444.42 | Formula | C20H21FN6O5 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 518048-05-0 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | MK-0518 | Smiles | CC1=NN=C(O1)C(=O)NC(C)(C)C2=NC(=C(C(=O)N2C)O)C(=O)NCC3=CC=C(C=C3)F | ||
Mechanism of Action
| Features |
The 1st approved human immunodeficiency virus type 1 (HIV-1) integrase inhibitor.
|
|---|---|
| Targets/IC50/Ki |
Integrase (S217Q PFV)
(Cell-free assay) 40 nM
Integrase (WT PFV)
(Cell-free assay) 90 nM
|
| In vitro |
PFV IN carrying the S217H substitution is 10-fold less susceptible to Raltegravir with IC50 of 900 nM. PFV IN displays 10% of WT activity and is inhibited by this compound with an IC50 of 200 nM, indicating a ~twofold decrease in susceptibility to the IN strand transfer inhibitor (INSTI) compared with WT IN. S217Q PFV IN is as sensitive to this compound as the WT enzyme. It is metabolized by glucuronidation, not hepatically. This compound has potent in vitro activity against HIV-1, with a 95% inhibitory concentration of 31?0 nM, in human T lymphoid cell cultures. It is also active against HIV-2 when tested in CEMx174 cells, with an IC95 of 6 nM. Its metabolism occurs primarily through glucuronidation. Drugs that are strong inducers of the glucuronidation enzyme, UGT1A1, significantly reduce its concentrations and should not be used. It exhibits weak inhibitory effects on hepatic cytochrome P450 activity. It does not induce CYP3A4 RNA expression or CYP3A4-dependent testosterone 6-β-hydroxylase activity. Its cellular permeativity is reduced in the presence of magnesium and calcium. This compound and related HIV-1 integrase (IN) strand transfer inhibitors (INSTIs efficiently block viral replication. In acutely infected human lymphoid CD4+ T-cell lines MT-4 and CEMx174, SIVmac251 replication is efficiently inhibited by this compound, which shows an EC90 in the low nanomolar range.
|
| Kinase Assay |
PFV integration assay
|
|
For quantitative strand transfer assays, donor DNA substrate is formed by annealing HPLC grade oligonucleotides 5′-GACTCACTATAGGGCACGCGTCAAAATTCCATGACA and 5′-ATTGTCATG GAATTTTGACGCGTGCCCTATAGTGAGTC. Reactions (40 μL) contains 0.75 μM PFV IN, 0.75 μM donor DNA, 4 nM (300 ng) supercoiled pGEM9-Zf(−) target DNA, 125 mM NaCl, 5 mM MgSO4, 4 μM ZnCl2, 10 mM DTT, 0.8% (vol/vol) DMSO, and 25 mM BisTris propane–HCl, pH 7.45. Raltegravir is added at indicated concentrations. Reactions are initiated by addition of 2 μL PFV IN diluted in 150 mM NaCl, 2 mM DTT, and 10 mM Tris-HCl, pH 7.4, and stopped after 1 hour at 37 °C by addition of 25 mM EDTA and 0.5% (wt/vol) SDS. Reaction products, deproteinized by digestion with 20 μg proteinase K for 30 minutes at 37 °C followed by ethanol precipitation, are separated in 1.5% agarose gels and visualized by staining with ethidium bromide. Integration products are quantified by quantitative real-time PCR, using Platinum SYBR Green qPCR SuperMix and three primers: 5′-CTACTTACTCTAGCTTCCCGGCAAC, 5′-TTCGCCAGTTAATAGTTTGCGCAAC, and 5′-GACTCACTATAGGGCACGCGT. PCR reactions (20 μL) contained 0.5 μM of each primer and 1 μL diluted integration reaction product. Following a 5-min denaturation step at 95 °C, 35 cycles are carried out in a CFX96 PCR instrument, using 10 seconds denaturation at 95 °C, 30 seconds annealing at 56 °C and 1 minutes extension at 68 °C. Standard curves are generated using serial dilutions of WT PFV IN reaction in the absence of this compound.
|
|
| In vivo |
Raltegravir induces viro-immunological improvement of nonhuman primates with progressing SIVmac251 infection. One non-human primate shows an undetectable viral load following this compound monotherapy.
|
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | CHOP / ATF-4 / XBP-1 / Lamin B |
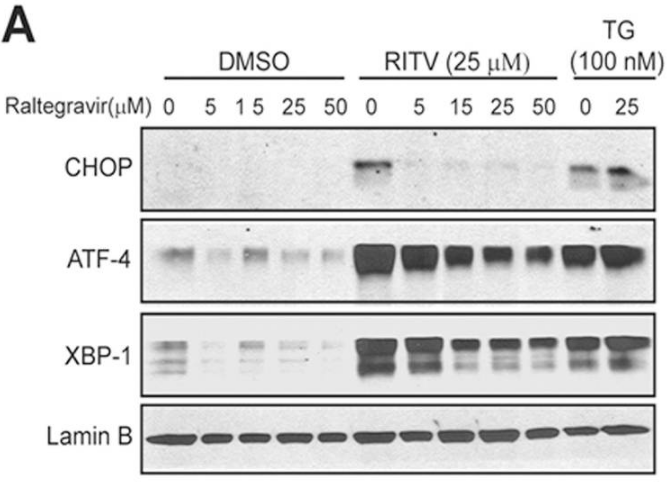
|
24625618 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT04513626 | Recruiting | HIV Infections |
Centre de Recherches et d''Etude sur la Pathologie Tropicale et le Sida |
September 15 2020 | Phase 2 |
| NCT03667547 | Completed | Human Immunodeficiency Virus (HIV) Infection |
Merck Sharp & Dohme LLC |
September 27 2018 | Phase 4 |
| NCT03174977 | Recruiting | HIV-1-infection |
University of California San Francisco|Merck Sharp & Dohme LLC |
April 1 2018 | Early Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































