research use only
PT2977 (Belzutifan) HIF-2α inhibitor
Cat.No.S8886
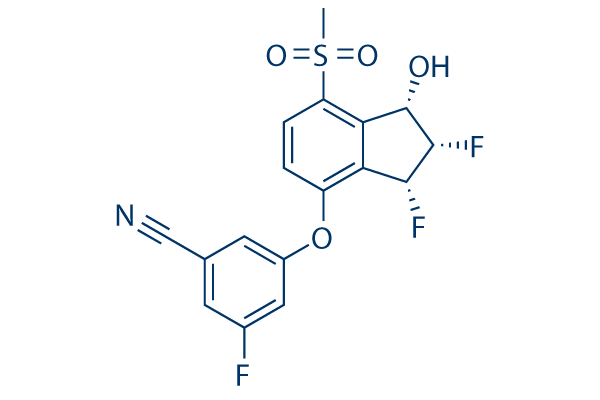
Chemical Structure
Molecular Weight: 383.34
Quality Control
| Related Targets | HSP JNK Antioxidant ROS eIF PERK IRE1 PKR ASK PDI |
|---|---|
| Other HIF Inhibitors | PT2399 PX-478 Dihydrochloride BAY 87-2243 KC7F2 Lificiguat (YC-1) IOX2 CAY10585 (LW 6) Molidustat (BAY 85-3934) PT2385 IDF-11774 |
Solubility
|
In vitro |
DMSO
: 77 mg/mL
(200.86 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 383.34 | Formula | C17H12F3NO4S |
Storage (From the date of receipt) | 3 years -20°C powder |
|---|---|---|---|---|---|
| CAS No. | 1672668-24-4 | -- | Storage of Stock Solutions |
|
|
| Synonyms | MK-6482 | Smiles | CS(=O)(=O)C1=C2C(C(C(C2=C(C=C1)OC3=CC(=CC(=C3)C#N)F)F)F)O | ||
Mechanism of Action
| Targets/IC50/Ki |
HIF-2α
9 nM
|
References |
|---|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT04994522 | Completed | End Stage Renal Disease|Renal Impairment |
Merck Sharp & Dohme LLC |
July 12 2022 | Phase 1 |
| NCT04846920 | Active not recruiting | Carcinoma Renal Cell |
Merck Sharp & Dohme LLC |
June 14 2021 | Phase 1 |
| NCT04627064 | Active not recruiting | Clear Cell Renal Cell Carcinoma |
Dana-Farber Cancer Institute|Eli Lilly and Company |
December 31 2020 | Phase 1 |
| NCT04489771 | Active not recruiting | Carcinoma Renal Cell |
Merck Sharp & Dohme LLC |
September 13 2020 | Phase 2 |
| NCT03634540 | Active not recruiting | Renal Cell Carcinoma (RCC)|Clear Cell Renal Cell Carcinoma (ccRCC)|Kidney Cancer|Renal Cancer|Renal Cell Carcinoma|Renal Cell Cancer Metastatic|Renal Cell Carcinoma Recurrent|Renal Cell Cancer Recurrent|Kidney |
Peloton Therapeutics Inc. a subsidiary of Merck & Co. Inc. (Rahway New Jersey USA) |
September 27 2018 | Phase 2 |
| NCT03401788 | Active not recruiting | VHL - Von Hippel-Lindau Syndrome|VHL Gene Mutation|VHL Syndrome|VHL Gene Inactivation|VHL-Associated Renal Cell Carcinoma|VHL-Associated Clear Cell Renal Cell Carcinoma |
Peloton Therapeutics Inc. a subsidiary of Merck & Co. Inc. (Rahway New Jersey USA) |
May 2 2018 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































