- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Polyinosinic acid-polycytidylic acid (Poly(I:C)) TLR3 Agonist
Cat.No.E0518
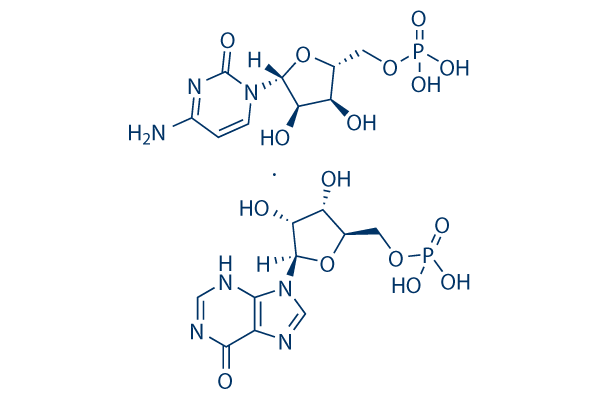
Chemical Structure
Molecular Weight: 671.40
Jump to
Quality Control
Batch:
Purity:
99.91%
99.91
Solubility
|
In vitro |
Water : 50 mg/mL
DMSO
: Insoluble
Ethanol : Insoluble |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 671.40 | Formula | C19H27N7O16P2 |
Storage (From the date of receipt) | 3 years -20°C powder |
|---|---|---|---|---|---|
| CAS No. | 24939-03-5 | -- | Storage of Stock Solutions |
|
|
| Synonyms | Polyinosinic-polycytidylic acid; Poly(I:C) | Smiles | NC1=NC(=O)N(C=C1)C2OC(CO[P;v5](O)(O)=O)C(O)C2O.OC3C(O)C(OC3CO[P;v5](O)(O)=O)[N]4C=NC5=C4NC=NC5=O | ||
Mechanism of Action
| References |
|---|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT02423863 | Completed | Melanoma|Head and Neck Cancer|Sarcoma|Non-Melanoma Skin Cancers |
Oncovir Inc.|National Institutes of Health (NIH)|Icahn School of Medicine at Mount Sinai|Bay Hematology Oncology|National Cancer Institute (NCI)|University of Missouri-Columbia|Chevy Chase Regional Cancer Care Associates LLC|Dermatologic Surgery Center of Washington LLC and Skin Cancer Treatment Center |
March 2015 | Phase 2 |
| NCT01727895 | Completed | Immunologic Deficiency Syndromes |
Radboud University Medical Center |
May 2013 | Not Applicable |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































