research use only
Licochalcone D NF-κB inhibitor
Cat.No.S6899
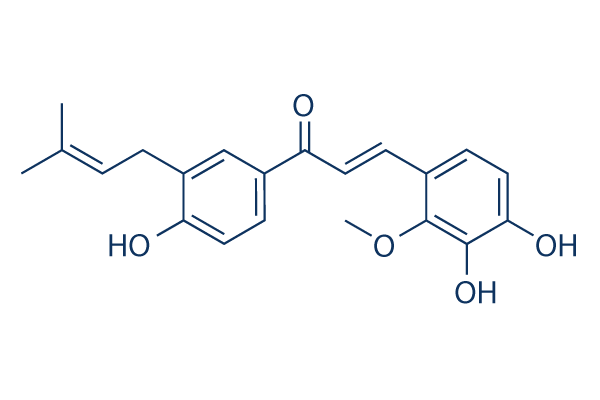
Chemical Structure
Molecular Weight: 354.40
Quality Control
| Related Targets | EGFR VEGFR PDGFR FGFR c-Met Src MEK CSF-1R FLT3 HER2 |
|---|---|
| Other NF-κB Inhibitors | DCZ0415 Omaveloxolone (RTA-408) BAY 11-7082 (BAY 11-7821) JSH-23 QNZ (EVP4593) Caffeic Acid Phenethyl Ester SC75741 DHA (Dihydroartemisinin) Withaferin A (WFA) Andrographolide |
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(282.16 mM)
Ethanol : 20 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 354.40 | Formula | C21H22O5 |
Storage (From the date of receipt) | 3 years -20°C powder |
|---|---|---|---|---|---|
| CAS No. | 144506-15-0 | -- | Storage of Stock Solutions |
|
|
Mechanism of Action
| Targets/IC50/Ki |
NF-κB
JAK2
EGFR
Met
Caspase
PARP
|
References |
|
|---|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06045416 | Not yet recruiting | Lyme Disease|Borrelia Infections |
University Children''s Hospital Zurich|Institute of Medical Microbiology University of Zurich Zurich Switzerland |
April 1 2024 | -- |
| NCT06003179 | Not yet recruiting | Lymphoma Large B-Cell Diffuse |
University Health Network Toronto |
April 2024 | Phase 1 |
| NCT06014762 | Recruiting | Diffuse Large B Cell Lymphoma|Follicular Lymphoma|Mantle Cell Lymphoma|Marginal Zone Lymphoma|Primary Mediastinal Large B-cell Lymphoma (PMBCL)|Chronic Lymphocytic Leukemia |
Poseida Therapeutics Inc.|Roche-Genentech |
April 2024 | Phase 1 |
| NCT06358859 | Not yet recruiting | Cardiometabolic Risk Factors|Diabetes|High Blood Pressure|Obesity|Nutrition Security |
Tufts University|National Institute on Minority Health and Health Disparities (NIMHD)|Tougaloo College Mississippi|Delta Health Center Mississippi|Reuben V. Anderson Center for Justice at Tougaloo |
April 2024 | Not Applicable |
| NCT05559801 | Not yet recruiting | Osteogenesis Imperfecta|Osteogenesis Imperfecta Type III |
Emory University |
April 2024 | Phase 1|Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































