research use only
L-Arginine HCl (L-Arg) NOS chemical
Cat.No.S3174
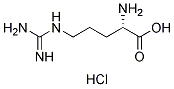
Chemical Structure
Molecular Weight: 210.66
Quality Control
Solubility
|
In vitro |
Water : 42 mg/mL
DMSO
: Insoluble
Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 210.66 | Formula | C6H14N4O2.HCl |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1119-34-2 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | (S)-(+)-Arginine hydrochloride | Smiles | C(CC(C(=O)O)N)CN=C(N)N.Cl | ||
Mechanism of Action
| In vitro |
L-Arginine HCl (L-Arg) supplementation (0.3 mM, 30 minutes) does not induce any significant increases in the peak NO concentration at low level of native LDL. However, at native LDL concentrations from 60-130 mg cholesterol/dL, NO concentration is 2 times higher than before treatment in bovine aortic endothelial cells. This compound results in a significant increase of NO production in n-LDL–treated cells as well as in oxidized -LDL–treated cells in the same cell type. It does not increase O2- concentration at low nativeLDL concentrations but reduces O2- production by 50% when incubated with n-LDL at concentrations >40 mg cholesterol/dL. Furthermore, it completely abolishes O2- production at every oxidized LDL dosage in bovine aortic endothelial cells. |
|---|---|
| In vivo |
L-Arginine HCl (L-Arg) (4 mg/kg/min for 1 hour) treatment decreases superoxide generation by cNOS while increasing NO accumulation in rabbit limb during ischemia/reperfusion. This compound prevents microvessel constriction in the reperfused muscle despite reduced but still apparent interstitial edema in rabbit limb, and results in a significant reduction of muscular reperfusion edema in rabbit limb. Its supplementation (0.1 g/kg, oral) significantly reduces pulmonary artery systolic pressure by a mean of 15.2% after 5 days of therapy in patients with sickle cell disease. Both L-Arginine and ornithine concentrations increased significantly after 5 days of oral supplementation in patients with sickle cell disease. It is associated with a decrease in cardiac index while stroke index is maintained in patients with severe sepsis. Resolution of shock at 72 hours is achieved by 40% and 24% of the patients in the L-Arginine and placebo cohorts, respectively. This compound (450 mg/kg during a 15-minute period) amplifies and sustains the hyperemia (38%) and increases absolute brain blood flow after eNOS upregulation by chronic simvastatin treatment (2 mg/kg subcutaneously, daily for 14 days) in SV-129 mice. |
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06265623 | Not yet recruiting | Chronic Obstructive Pulmonary Disease (COPD) |
Universitätsklinikum Hamburg-Eppendorf |
March 1 2024 | -- |
| NCT06244758 | Recruiting | Type2diabetes |
University of Erlangen-Nürnberg Medical School|Bayer |
January 18 2024 | Phase 3 |
| NCT06137833 | Not yet recruiting | Fatigue|Breast Cancer |
Pharmanutra S.p.a.|Latis S.r.l. |
November 27 2023 | Not Applicable |
| NCT05934318 | Not yet recruiting | Pregnancy|Malaria|Nutrition|Placental Development|Preterm Birth|Fetal Growth Restriction |
Liverpool School of Tropical Medicine|Kenya Medical Research Institute|University of Toronto|Telethon Kids Institute |
September 30 2023 | Not Applicable |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































