research use only
PluriSIn #1 (NSC 14613) SCD inhibitor
Cat.No.S8076
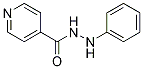
Chemical Structure
Molecular Weight: 213.24
Quality Control
| Related Targets | Dehydrogenase HSP Transferase P450 (e.g. CYP17) PDE phosphatase PPAR Vitamin Carbohydrate Metabolism Mitochondrial Metabolism |
|---|---|
| Other SCD Inhibitors | MK-8245 A939572 MF-438 Aramchol CAY10566 CVT-11127 |
Solubility
|
In vitro |
DMSO
: 43 mg/mL
(201.65 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 213.24 | Formula | C12H11N3O |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 91396-88-2 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | C1=CC=C(C=C1)NNC(=O)C2=CC=NC=C2 | ||
Mechanism of Action
| Targets/IC50/Ki |
SCD1
|
|---|---|
| In vitro |
PluriSIn #1 (NSC 14613) has a robust, rapid, and selective cytotoxic effect toward hPSCs, with apoptosis as the central cell death mechanism it activates. This compound leads to ER stress in hPSCs, inducing a ~30% decrease in protein synthesis at 20 μM. It also causes a ~65% decrease in stearoyl-coA desaturase (SCD1) activity and prevents teratoma formation from undifferentiated hPSCs. PluriSIns also inhibits mPSCs and hinders mouse embryonic development.
|
| Kinase Assay |
SCD1 activity assays
|
|
Cells are plated in 6-well plates at a density of 50k to 100k cells per well. 24 h later, 20 μM PluriSIn #1 (NSC 14613) or 0.2% DMSO-control are added to the cells. After 12 h of incubation at 37 ℃, 5% CO2, the old medium is removed, cells are washed with PBS, and new medium containing 2.3 μM of 0.75 UCi [1-14C] Stearic Acid is added. The cells are incubated for up to 4 h at 37 ℃, 5% CO2.
After the incubation period, the medium is discarded and the cells are washed 3 times with 2 mL of PBS. 2 mL of the mixture n-hexane: isopropanol (3:2 v:v) are added, and the cells are incubated for 30 min at 37 ℃, 5% CO2. 2 mL Folch solution (chloroform: methanol,2:1,v:v) are subsequently added. The liquid is transferred to tubes for phase partition by adding 1 mL water. The lower organic phase is evaporated and used for lipid saponification and TLC separation of the free [1-14C] Stearic Acid (substrate) and [1-14C] Oleic Acid (formed product). Lipids extracted from the cells are applied to TLC plates previously immersed in 10% NO3 Ag and activated at 120℃x60 min. Unlabeled stearic and oleic acid are added to each application point as carriers and as internal standards for identification. The plates are run with a solvent mixture of Chloroform:MeOH:AcH:DDW (90:8:1:0.8). The free fatty acids are detected by U.V. after spraying the TLC with a 2',7',dichlorofluorescein solution. The spots corresponding to stearic and oleic acid are scraped and the radioactivity counted in a scintillating counter. SCD1 desaturase activity is calculated from the percent conversion of substrate to product and the conversion to pmol/min/106 cells.
|
References |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































