- Bioactive Compounds
- By Signaling Pathways
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- Compound Libraries
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-Ⅱ
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Plant Extract Library
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at [email protected] to customize your library.
You could select:
- Antibodies
- Bioreagents
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- Protein Assay
- Protein A/G Magnetic Beads for IP
- Anti-Flag magnetic beads
- Anti-Flag Affinity Gel
- Anti-Myc magnetic beads
- Anti-HA magnetic beads
- Poly FLAG Peptide lyophilized powder
- Protease Inhibitor Cocktail
- Protease Inhibitor Cocktail (EDTA-Free, 100X in DMSO)
- Phosphatase Inhibitor Cocktail (2 Tubes, 100X)
- Cell Biology
- Cell Counting Kit-8 (CCK-8)
- Animal Experiment
- Mouse Direct PCR Kit (For Genotyping)
- New Products
- Contact Us
Ispinesib (SB-715992)
Synonyms: CK0238273
Ispinesib (SB-715992, CK0238273) is a potent, specific and reversible inhibitor of kinesin spindle protein (KSP) with Ki app of 1.7 nM in a cell-free assay, no inhibition to CENP-E, RabK6, MCAK, MKLP1, KHC or Kif1A. Ispinesib induces mitotic arrest and apoptotic cell death.
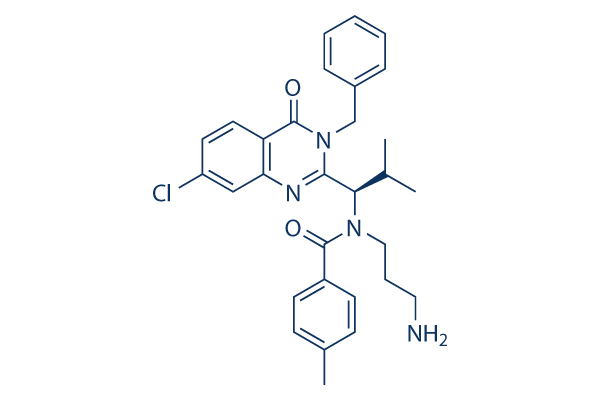
Ispinesib (SB-715992) Chemical Structure
CAS: 336113-53-2
Selleck's Ispinesib (SB-715992) has been cited by 26 Publications
4 Customer Reviews
Purity & Quality Control
Batch:
Purity:
99.97%
99.97
Ispinesib (SB-715992) Related Products
| Related Targets | Kinesin-12 | Click to Expand |
|---|---|---|
| Related Compound Libraries | Kinase Inhibitor Library Angiogenesis Related compound Library TGF-beta/Smad compound library Cytoskeletal Signaling Pathway Compound Library Anti-Cardiovascular Disease Compound Library | Click to Expand |
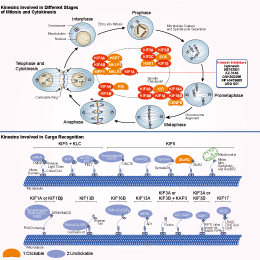
Signaling Pathway
Choose Selective Kinesin Inhibitors
Cell Data
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| HCT116 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT116 cells after 72 hrs by Alamar blue assay, GI50=0.025μM | 22248262 | ||
| hTERT-HME1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human hTERT-HME1 cells after 72 hrs by Alamar blue assay, GI50=0.032μM | 22248262 | ||
| K562 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human K562 cells after 72 hrs by Alamar blue assay, GI50=0.071μM | 22248262 | ||
| BxPC3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human BxPC3 cells after 72 hrs by Alamar blue assay, GI50=0.08μM | 22248262 | ||
| NCI-H1299 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human NCI-H1299 cells after 72 hrs by Alamar blue assay, GI50=0.082μM | 22248262 | ||
| LNCAP | Growth inhibition assay | 72 hrs | Growth inhibition of human LNCAP cells after 72 hrs by Alamar blue assay, GI50=0.022μM | 23394180 | ||
| HCT116 | Growth inhibition assay | 72 hrs | Growth inhibition of human HCT116 cells after 72 hrs by Alamar blue assay, GI50=0.025μM | 23394180 | ||
| PC3 | Growth inhibition assay | 72 hrs | Growth inhibition of human PC3 cells after 72 hrs by Alamar blue assay, GI50=0.05μM | 23394180 | ||
| BxPC3 | Growth inhibition assay | 72 hrs | Growth inhibition of human BxPC3 cells after 72 hrs by Alamar blue assay, GI50=0.08μM | 23394180 | ||
| NCI-H1299 | Growth inhibition assay | 72 hrs | Growth inhibition of human NCI-H1299 cells after 72 hrs by Alamar blue assay, GI50=0.082μM | 23394180 | ||
| LXFS 538 | Antitumor assay | 6 mg/kg | Antitumor activity against human LXFS 538 cells xenografted in NMRI nu/nu mouse assessed as tumor regression at 6 mg/kg, ip measured on day 13 | 23394180 | ||
| HeLa | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HeLa cells after 48 hrs by MTT assay, IC50=1μM | 24184776 | ||
| MCF7 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MCF7 cells after 48 hrs by MTT assay, IC50=1.3μM | 24184776 | ||
| HCT116 | Growth inhibition assay | 72 hrs | Growth inhibition of human HCT116 cells after 72 hrs by MTS assay, IC50=0.0011μM | 26396688 | ||
| TC32 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for TC32 cells | 29435139 | |||
| DAOY | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for DAOY cells | 29435139 | |||
| SJ-GBM2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SJ-GBM2 cells | 29435139 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 29435139 | |||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 29435139 | |||
| Saos-2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Saos-2 cells | 29435139 | |||
| LAN-5 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for LAN-5 cells | 29435139 | |||
| BT-12 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-12 cells | 29435139 | |||
| OHS-50 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for OHS-50 cells | 29435139 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for A673 cells) | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for SK-N-MC cells | 29435139 | |||
| BT-12 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for BT-12 cells | 29435139 | |||
| DAOY | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for DAOY cells | 29435139 | |||
| BT-37 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for BT-37 cells | 29435139 | |||
| TC32 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for TC32 cells | 29435139 | |||
| MG 63 (6-TG R) | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for MG 63 (6-TG R) cells | 29435139 | |||
| U-2 OS | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for U-2 OS cells | 29435139 | |||
| Rh41 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for Rh41 cells | 29435139 | |||
| RD | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for RD cells | 29435139 | |||
| Rh30 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for Rh30 cells | 29435139 | |||
| Saos-2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for Saos-2 cells | 29435139 | |||
| SK-N-SH | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for SK-N-SH cells | 29435139 | |||
| Click to View More Cell Line Experimental Data | ||||||
Biological Activity
| Description | Ispinesib (SB-715992, CK0238273) is a potent, specific and reversible inhibitor of kinesin spindle protein (KSP) with Ki app of 1.7 nM in a cell-free assay, no inhibition to CENP-E, RabK6, MCAK, MKLP1, KHC or Kif1A. Ispinesib induces mitotic arrest and apoptotic cell death. | ||
|---|---|---|---|
| Features | An allosteric, potent, specific, and reversible inhibitor of the mitotic kinesin spindle protein (KSP) (HsEg5). | ||
| Targets |
|
| In vitro | ||||
| In vitro | Ispinesib is a potent, allosteric, reversible, and specific inhibitor of KSP, which changes the binding property of KSP to microtubules and disturbs its movement by inhibiting ADP release without altering the release of the KSP-ADP complex from the microtubule. [1] Ispinesib shows potent cytotoxic activity in a panel of tumor cell lines, including Colo205, Colo201, HT-29, M5076, Madison-109, and MX-1, with IC50 of 1.2 nM to 9.5 nM. [2] In PC-3 prostate cancer cells, Ispinesib (15 nM and 30 nM) blocks cell proliferation and induces apoptosis by regulating the expression levels of genes that controls apoptosis, cell proliferation, cell cycle, and cell signaling, such as EGFR, p27, p15, and IL-11. [3] In a panel of 53 breast cell lines, Ispinesib (7.4 nM–600 nM) demonstrates broad inhibitory activity. In BT-474 and MDA-MB-468 cells, Ispinesib (150 nM) induces apoptosis, as revealed by a higher proportion of apoptotic cells, lower antiapoptotic Bcl-XL level, and higher proapoptotic Bax and Bid levels. [4] |
|||
|---|---|---|---|---|
| Kinase Assay | Steady-State Kinetic Analysis of Human KSP ATPase Activity and Inhibition by Ispinesib | |||
| Kinesin specificity analysis is carried out using a pyruvate kinase-lactate dehydrogenase detection system that couples the production of ADP to oxidation of NADH. Absorbance changes are monitored at 340 nm. Steady-state studies using nanomolar concentrations of KSP are performed using a sensitive fluorescence-based assay utilizing a pyruvate kinase, pyruvate oxidase, and horseradish peroxidase (HRP) coupled detection system that couples the generation of ADP to oxidation of Amplex Red to fluorescent resorufin. Generation of resorufin is monitored by fluorescence (λexcitation = 520 nm and λemission = 580 nm). Steady-state biochemical experiments are performed in PEM25 buffer [25 mM Pipes-K+ (pH 6.8), 2 mM MgCl2, 1 mM EGTA] supplemented with 10 µM paclitaxel for experiments involving microtubules. The IC50 for steady-state inhibition is determined at 500 µM ATP, 5 µM Microtubules, and 1 nM KSP in PEM25 buffer. Ki app (apparent inhibitor dissociation constant) values of Ispinesib are extracted from the dose-response curves, with explicit correction for enzyme concentration by using the Morrison equation.Inhibitor modality (e.g., competitive, noncompetitive, uncompetitive, or mixed) under steady-state conditions is determined by measuring the effect of inhibitor concentration on initial velocity as a function of substrate concentrations. Data are fit using equations in GraFit to velocity equations for the various modes of inhibition. | ||||
| Cell Research | Cell lines | Breast cancer cells, including MCF-7, HCC1954, MDA-MB-468, and KPL4 | ||
| Concentrations | 0.085 nM–33 µM | |||
| Incubation Time | 72 hours | |||
| Method | Cells are plated in log phase of growth in 96-well plates and treated with Ispinesib for 72 hours. Then, cell growth is measured using CellTiter-Glo, and luminescence is detected using BioTek FLx800. Data are analyzed and the IC50 value, defined as the drug concentration that results in 50% growth inhibition relative to control, is calculated. |
|||
| In Vivo | ||
| In vivo |
Ispinesib (4.5 mg/kg–15 mg/kg) exhibits inhibitory effects against Colo205, Colo201, HT-29, but not MX-1 cells, in mouse xenograft models. SB-715992 (6 mg/kg–10 mg/kg ) also inhibits murine solid tumors, including Madison 109 lung carcinoma, M5076 sarcoma, as well as L1210 and P388 leukemias. [2] In mice xenograft models of breast cancer cells MCF-7, HCC1954, MDA-MB-468, and KPL4, Ispinesib (8 mg/kg–10 mg/kg) inhibits tumor growth. [4] |
|
|---|---|---|
| Animal Research | Animal Models | Nude (nu/nu) mice models of MCF7, KPL4, and HCC1954 cells; severe combined immunodeficient (SCID) mice model of MDA-MB-468 cells; |
| Dosages | 10 mg/kg for nude mice or 8 mg/kg for SCID mice | |
| Administration | Intraperitoneal injection on a q4d× schedule (3 doses, every 4 day | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT00607841 | Terminated | Breast Neoplasms |
Cytokinetics |
December 2007 | Phase 1 |
| NCT00354250 | Completed | Recurrent Renal Cell Cancer|Stage III Renal Cell Cancer|Stage IV Renal Cell Cancer |
National Cancer Institute (NCI) |
May 2006 | Phase 2 |
| NCT00097409 | Completed | Ovarian Cancer |
GlaxoSmithKline |
December 2004 | Phase 2 |
| NCT00119171 | Completed | Solid Tumor Cancer |
GlaxoSmithKline |
November 2004 | Phase 1 |
Chemical Information & Solubility
| Molecular Weight | 517.06 | Formula | C30H33ClN4O2 |
| CAS No. | 336113-53-2 | SDF | Download Ispinesib (SB-715992) SDF |
| Smiles | CC1=CC=C(C=C1)C(=O)N(CCCN)C(C2=NC3=C(C=CC(=C3)Cl)C(=O)N2CC4=CC=CC=C4)C(C)C | ||
| Storage (From the date of receipt) | |||
|
In vitro |
DMSO : 103 mg/mL ( (199.2 mM); Moisture-absorbing DMSO reduces solubility. Please use fresh DMSO.) Ethanol : 103 mg/mL Water : Insoluble |
Molecular Weight Calculator |
|
In vivo Add solvents to the product individually and in order. |
In vivo Formulation Calculator |
||||
Preparing Stock Solutions
Molarity Calculator
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Tech Support
Answers to questions you may have can be found in the inhibitor handling instructions. Topics include how to prepare stock solutions, how to store inhibitors, and issues that need special attention for cell-based assays and animal experiments.
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
* Indicates a Required Field
Tags: buy Ispinesib (SB-715992) | Ispinesib (SB-715992) supplier | purchase Ispinesib (SB-715992) | Ispinesib (SB-715992) cost | Ispinesib (SB-715992) manufacturer | order Ispinesib (SB-715992) | Ispinesib (SB-715992) distributor