Rationale for Adjudication: Why Do Central Adjudication?
Independent and consistent adjudication of events using uniformly applied endpoint definitions enhances freedom from bias and the interpretability of study results. Medical devices require evaluation during both pre-approval and post-approval phases to assess safety and effectiveness. Although study investigators are responsible for data submission, inherent bias may cause over- or under-reporting of events. For researchers developing new therapies, utilizing Immunology & Inflammation related Inhibitors requires similarly rigorous endpoint monitoring to distinguish therapeutic effects from inflammatory adverse events.
1. Limit Bias
Bias at the investigative site arises from several factors. An investigator using the investigational product may be an enthusiast, influencing the interpretation of an event's relatedness to the device. Financial or scientific relationships with manufacturers can exaggerate this. Furthermore, site investigators might interpret complication-related events erroneously due to direct involvement in care. Coding errors influenced by reimbursement incentives—such as assigning "heart failure" as a discharge diagnosis without supporting clinical trial criteria—can confound efforts to document bona-fide endpoints.
2. Standardized Definitions
The lack of pre-specified definitions in global trials often leads to variability, as seen in the early SOLVD trials where site-reported outcomes for cause-specific mortality differed from central adjudication results. Establishing uniform event definitions, such as those for Interleukins Inhibitors pathways in inflammatory disease trials, allows for better cross-trial comparisons. Standardized definitions ensure that worsening heart failure focuses on therapy escalation and clinical signs rather than just insurance claim coding.
When is Adjudication Appropriate?
Independent adjudication is useful in all studies where clinical endpoints require interpretation of clinical information. It is critical in pivotal studies of novel technology with inadequate masking, as is common in device trials. The CHARM PRESERVED trial illustrates this: a benefit with candesartan was seen with investigator-adjudicated events but was not found with CEC adjudication. This increased rigor can prevent promising therapies from being erroneously pursued or abandoned.
Best Adjudication Practices
The Cardiac Safety Research Consortium discussion highlighted that all Clinical Events Committees (CECs) should operate under a pre-approved charter. Key elements include:
- Qualifications of CEC members: Members must possess collective clinical and procedural expertise. In global studies, familiarity with regional practice patterns is essential.
- Independence: Adjudicators must not receive direct financial benefit from the sponsor and cannot serve as investigators at study sites.
- Endpoint Definitions: Definitions should follow criteria established by professional societies or regulatory references like the FDA Standardized Definitions for Cardiovascular and Stroke Endpoint Events.
- Event Identification: This requires systematic screening of safety summaries, lab data, and case report forms to prevent under-reporting by sites.
Practical Issues in Device Adjudication
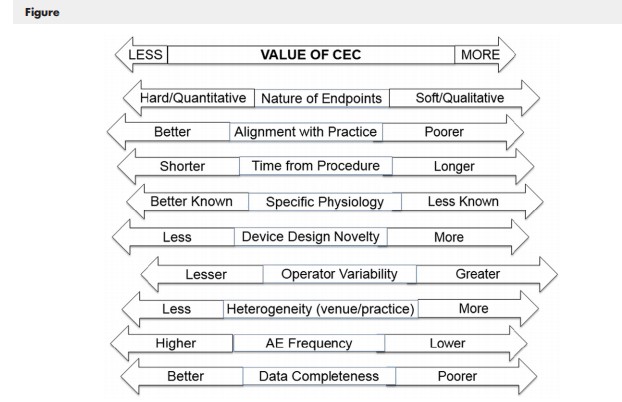
Factors determining the value of a CEC in medical device investigational plans include:
| Factor | Considerations for Implementation |
|---|---|
| Nature of Study Endpoints | Straightforward events like death vs. complex events like stroke or heart failure which require judgment. |
| Alignment with Practice | Endpoints requiring data beyond standard clinical practice (e.g., specialized imaging) increase the value of adjudication. |
| Operator Variability | Device performance relies on operator skill; adjudication is essential for accurate attribution of events to the device vs. technique. |
| Device Design Factors | Novel products (e.g., drug-coated balloons) may introduce new types of adverse events (AEs) requiring careful evaluation. |
| Completeness of Data | CEC adjudication can provide precision even when serial biomarker measurements are challenging to collect. |
Future Directions: Real-World Evidence (RWE)
FDA’s guidance on "Use of Real-World Evidence to Support Regulatory Decision-Making for Medical Devices" clarifies how RWE supports pre-market clearance and post-market safety monitoring. Adjudication remains vital in this era to maintain appropriate standards for patient safety while facilitating timely access to new medical technologies.
References & Further Reading
- Seltzer JH, et al. Use of endpoint adjudication... Rationale and best practices. Am Heart J 2017;190:76-85.
- FDA Guidance: Use of Real-World Evidence to Support Regulatory Decision-Making for Medical Devices.
- European Commission Guideline on Medical Devices: Standard Definitions.