research use only
Deferasirox (ICL670) CYP1A2 Inhibitor
Cat.No.S1712
Chemical Structure
Molecular Weight: 373.36
Quality Control
| Related Targets | HDAC Caspase Proteasome Secretase MMP HCV Protease Cysteine Protease Tyrosinase DPP HIV Protease |
|---|---|
| Other P450 (e.g. CYP17) Inhibitors | Apigenin Baicalein Avasimibe Naringenin Diosmetin Alizarin Sodium Danshensu Naringin Benzbromarone Orteronel |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| MDA-MB-231 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human MDA-MB-231 cells after 96 hrs by MTT assay, IC50=4μM | 20005708 | ||
| MIAPaCa2 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human MIAPaCa2 cells after 96 hrs by MTT assay, IC50=10μM | 20005708 | ||
| SK-N-MC | Toxicity assay | Toxicity in human SK-N-MC cells by MTT method, IC50=20.54μM | 20041672 | |||
| Sf9 | Function assay | 20 mins | Inhibition of human MRP4 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and GSH measured after 20 mins by membrane vesicle transport assay, IC50=36.5μM | 23956101 | ||
| SJ-GBM2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SJ-GBM2 cells | 29435139 | |||
| BT-37 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-37 cells | 29435139 | |||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 29435139 | |||
| OHS-50 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for OHS-50 cells | 29435139 | |||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 75 mg/mL
(200.87 mM)
Ethanol : 2 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 373.36 | Formula | C21H15N3O4 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 201530-41-8 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | CGP-72670, ICL-670 | Smiles | C1=CC=C(C(=C1)C2=NN(C(=N2)C3=CC=CC=C3O)C4=CC=C(C=C4)C(=O)O)O | ||
Mechanism of Action
| In vitro |
Deferasirox effectively chelates iron from Rhizopus oryzae and demonstrates cidal activity in vitro against 28 of 29 clinical isolates of Mucorales at concentrations well below clinically achievable serum levels. This compound incubation induces a significant inhibition of NF-κB activity and a cytoplasmic sequestration of its active subunit p65 in an inactive form in 28 of 40 peripheral blood samples. It inhibits three human myeloid cell lines (K562, U937, and HL60) with IC50 of 17-50 mM. The chemical is cidal in vitro against A. fumigatus, with an MIC and MFC of 25 and 50 mg/L, respectively. |
|---|---|
| In vivo |
Deferasirox significantly improves survival and decreased tissue fungal burden in diabetic ketoacidotic or neutropenic mice with mucormycosis, with an efficacy similar to that of liposomal amphotericin B. This compound treatment also enhances the host inflammatory response to mucormycosis. It synergistically improves survival and reduces tissue fungal burden when combined with liposomal amphotericin B. This chemical administered p.o. to rats is absorbed to at least 75%, and the bioavailability is 26%. It is present in the blood circulation mainly in the unchanged form and as its iron complex, Fe(deferasirox)2, after intravenous and p.o. administration. The compound is 99.2% bound to plasma proteins. It monotherapy modestly prolongs survival of mice with IPA. |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | CDK2 / CDK4 / CDK6 / Cyclin A / Cyclin B / Cyclin D1 / Cyclin E / p53 / p27 / p21 TFR1 / Ferroportin Pro-caspase-3 / Pro-caspase-8 / Pro-caspase-9 / BAX / NDRG1 / c-Myc / p-mTOR |
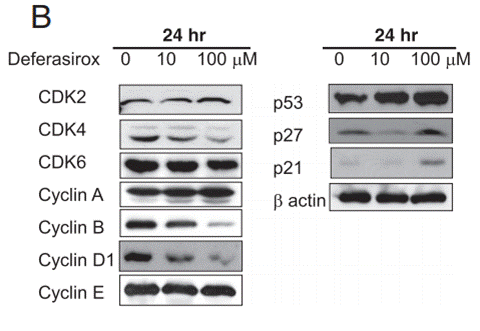
|
26965928 |
| Immunofluorescence | Bax / TOM22 Cytochrome c |
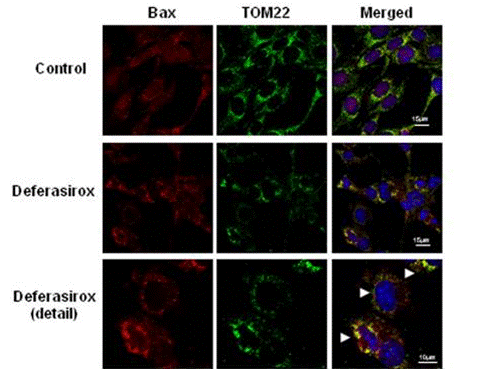
|
28139717 |
| Growth inhibition assay | Cell viability Cell viability |
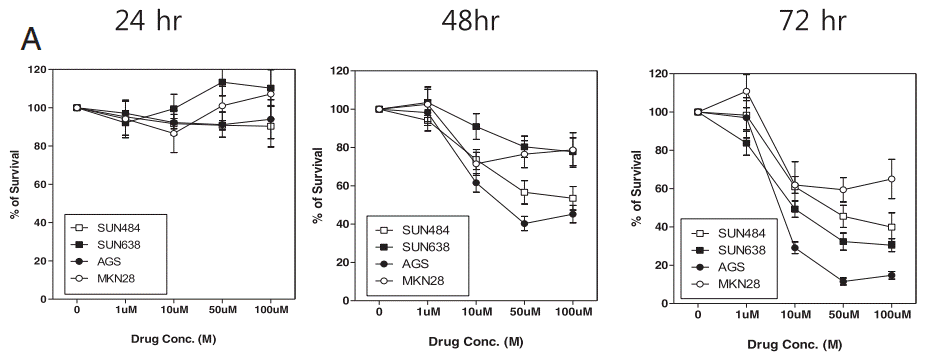
|
26965928 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT04423237 | Recruiting | Iron Overload |
University of Pisa|IRCCS Burlo Garofolo|University of Genova |
September 30 2020 | -- |
| NCT03920657 | Terminated | Myelodysplastic Syndromes |
Fondazione Italiana Sindromi Mielodisplastiche-ETS |
October 4 2019 | Phase 2 |
| NCT03372083 | Completed | Iron Overload |
Novartis Pharmaceuticals|Novartis |
January 16 2018 | Phase 4 |
| NCT02663752 | Terminated | Myelodysplastic Syndrome |
Novartis Pharmaceuticals|Novartis |
May 30 2016 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































