research use only
Silymarin Antioxidant chemical
Cat.No.S2358
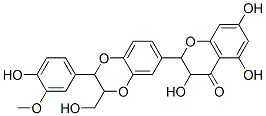
Chemical Structure
Molecular Weight: 482.44
Quality Control
| Related Targets | EGFR VEGFR JAK PDGFR FGFR Src HIF FLT FLT3 HER2 |
|---|---|
| Other Antioxidant Inhibitors | Elamipretide (SS-31, MTP-131) Tangeretin (+)-Catechin Hydroxygenkwanin Oleuropein (-)Epicatechin Syringic acid Cocoa Extract Clematis apiifolia Extract Parsley Extract |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 482.44 | Formula | C25H22O10 |
Storage (From the date of receipt) | 3 years -20°C powder (seal) |
|---|---|---|---|---|---|
| CAS No. | 65666-07-1 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | Silybin B | Smiles | COC1=C(C=CC(=C1)C2C(OC3=C(O2)C=C(C=C3)C4C(C(=O)C5=C(C=C(C=C5O4)O)O)O)CO)O | ||
Mechanism of Action
| In vitro |
Silymarin (Silybin B), a polyphenolic flavonoid extracted from the seeds of Silybum marianum or milk thistle, is used in the prevention and treatment of liver diseases and primary liver cancer. The mechanisms of action of this compound involve different biochemical events, such as the stimulation of the synthetic rate of ribosomal RNA (rRNA) species through stimulation of polymerase I and rRNA transcription, protecting the cell membrane from radical-induced damage and blockage of the uptake of toxins such as alpha-amanitin. This chemical can also provide substantial protection against different stages of UVB-induced carcinogenesis, possibly via its strong antioxidant properties. |
|---|---|
| In vivo |
LD50: Mice 391.4mg/kg (i.v.); Rats 370.1mg/kg (i.v.); Rabbits 142.3mg/kg (i.v.) |
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05099601 | Unknown status | Melasma |
Assiut University |
May 2022 | Phase 4 |
| NCT04434404 | Completed | Breast Cancer |
Horus University |
September 10 2018 | Phase 4 |
| NCT03216668 | Unknown status | Liver Function Failure |
Nhat Nhat Pharmaceutical Company |
June 15 2017 | Phase 3 |
| NCT02006498 | Completed | Non-alcoholic Fatty Liver Disease |
University of Malaya|Rottapharm |
June 2012 | Phase 2 |
| NCT01018615 | Completed | Chronic Hepatitis C|Oxidative Stress |
University of North Carolina Chapel Hill|National Center for Complementary and Integrative Health (NCCIH)|National Institutes of Health (NIH) |
November 2009 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































