research use only
Phenothiazine Dopamine Receptor antagonist
Cat.No.S4251
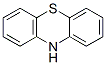
Chemical Structure
Molecular Weight: 199.27
Quality Control
| Related Targets | Adrenergic Receptor AChR 5-HT Receptor COX Calcium Channel Histamine Receptor GABA Receptor TRP Channel Cholinesterase (ChE) GluR |
|---|---|
| Other Dopamine Receptor Inhibitors | MPTP Hydrochloride Trifluoperazine Trifluoperazine 2HCl Penfluridol Sulpiride SCH-23390 hydrochloride Levosulpiride Domperidone SKF38393 HCl Rotundine |
Solubility
|
In vitro |
DMSO
: 40 mg/mL
(200.73 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 199.27 | Formula | C12H9NS |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 92-84-2 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | ENT 38 | Smiles | C1=CC=C2C(=C1)NC3=CC=CC=C3S2 | ||
Mechanism of Action
| Targets/IC50/Ki |
D2 receptor
|
|---|---|
| In vitro |
Phenothiazines mostly substitutes at position 10 with the dialkylaminoalkyl groups and additionally at position 2 with small groups exhibit valuable activities such as neuroleptic, antiemetic, antihistaminic, antipuritic, analgesic and antihelmintic. 2-trifluoromethyl-10-(4-aminobutyl)phenothiazine inhibits S. cerevisiae strains and T. mentagrophites with MIC of 0.4 μg/mL and 1.5 μg/mL, respectively. 10-carbamoylalkyl derivatives shows significant activities against Gram-positive Bacillus subtilis with MIC’s in the range of 7.8 μg/mL–30 μg/mL. The tetracyclic compounds (modified with the naphthoquinone ring) shows significant actibacterial activity against S. aureus with the MIC50 of 12.5 μg/mL. These compounds with the butylene linker are more effective than with the propylene linker, the 2-chloro-10-chloroethylureidobutyl derivative giving GI50 of 1.4 μM and 1.6 μM against 4 leukemia cell lines and 7 colon cancer cell lines. 10-Amino(hydroxy)propyl derivatives (5 μM) induces a marked G2/M phase of cell-cycle arrest followed by cell death in human transformed WI38VA cells after 2-day incubation. This class of drugs undergo extensive metabolism in the body before being excreted, mainly ring hydroxylation, ring sulphoxidation, N-demethylation, N-oxidation, sulphate and glucuronide conjugation. They have considerably lower binding affinities to α2-adrenoceptors than to dopamine D2 receptors and al-adrenoceptors. These compounds have significant in vitro activity against susceptible, polydrug- and multidrug-resistant strains of M. tuberculosis, as well as enhancing the activity of some agents employed for first-line treatment.
|
References |
|
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































