research use only
Zolmitriptan 5-HT Receptor agonist
Cat.No.S1649
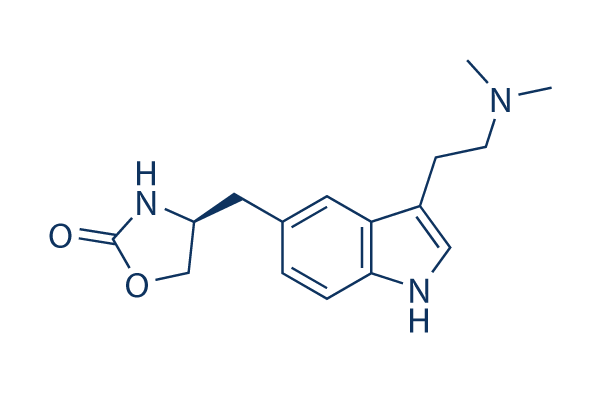
Chemical Structure
Molecular Weight: 287.36
Quality Control
| Related Targets | Adrenergic Receptor AChR COX Calcium Channel Histamine Receptor Dopamine Receptor GABA Receptor TRP Channel Cholinesterase (ChE) GluR |
|---|---|
| Other 5-HT Receptor Inhibitors | Puerarin WAY-100635 Maleate Serotonin (5-HT) HCl BRL-15572 Dihydrochloride SB269970 HCl Ketanserin RS-127445 Azacyclonol Nuciferine Flopropione |
Solubility
|
In vitro |
DMSO
: 57 mg/mL
(198.35 mM)
Ethanol : 57 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 287.36 | Formula | C16H21N3O2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 139264-17-8 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | BW-311C90 | Smiles | CN(C)CCC1=CNC2=C1C=C(C=C2)CC3COC(=O)N3 | ||
Mechanism of Action
| Targets/IC50/Ki |
5-HT
|
|---|---|
| In vitro |
Zolmitriptan produces concentration-dependent contractions of primate basilar artery and human epicardial coronary artery rings. This compound displays high affinity at human recombinant 5-HT1D (formerly 5-HT1D alpha) and 5-HT1B (formerly 5-HT1D beta) receptors in transfected CHO-K1 cell membranes. It increases I(K) in a concentration-dependent manner (maximum increase 16.3%) with a pD(2) value of 7.03 in C6 glioma cells expressing recombinant human 5-HT(1B) receptor. This compound-induced increases in I(K) are prevented by the calcium chelator, EGTA (5 mM) when included in the patch pipette in C6 cells expressing cloned human 5-HT(1B) receptors. |
| In vivo |
Zolmitriptan (3-30 mg/kg, i.v.) administrated ten minutes before unilateral electrical stimulation of the trigeminal ganglion causes a dose-dependent inhibition of [125I]-albumin extravasation within the ipsilateral dura mater in anaesthetized guinea-pigs. This compound (10-1000 mg/kg, i.v.) selectively reduces arteriovenous-anastomotic (AVA) conductance producing a maximum decrease of 92.5%. It also produces a modest reduction in extra-cerebral conductance (23.9% maximum reduction at 30 mg/kg, i.v.), but is without effect on cerebral conductance. This chemical (1-30 mg/kg, i.v.) produces dose-dependent decreases in ear microvascular conductance (15% to 60%) which mirror decreases in carotid arterial conductance in anaesthetised cats. It exerts behaviorally specific anti-aggressive effects in mice. This compound also decreases alcohol-heightened aggression with equal efficacy in mice. |
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06074016 | Completed | Healthy |
Parc de Salut Mar|Food and Drug Administration (FDA) |
July 12 2023 | Phase 1 |
| NCT05019430 | Recruiting | Cocaine Use Disorder |
William Stoops|National Institute on Drug Abuse (NIDA)|University of Kentucky |
October 15 2021 | Early Phase 1 |
| NCT01085123 | Completed | Migraine |
AstraZeneca |
May 2010 | Phase 1 |
| NCT00637286 | Completed | Migraine |
AstraZeneca |
July 2004 | Phase 4 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































