research use only
Tebipenem Pivoxil (L084) Carbapenem Antibiotic
Cat.No.S2159
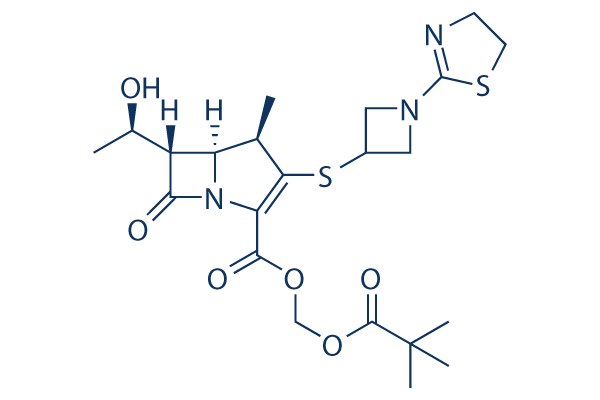
Chemical Structure
Molecular Weight: 497.63
Quality Control
| Related Targets | Integrase Bacterial Anti-infection Fungal Antiviral COVID-19 Parasite Reverse Transcriptase HIV HCV Protease |
|---|---|
| Other Antibiotics Inhibitors | G418 Sulfate (Geneticin) Puromycin Nanchangmycin Sitafloxacin Hydrate Gamithromycin Thiamphenicol Fusidine Tildipirosin Spiramycin Nadifloxacin |
Solubility
|
In vitro |
DMSO
: 99 mg/mL
(198.94 mM)
Ethanol : 87 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 497.63 | Formula | C22H31N3O6S2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 161715-24-8 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | L-084, ME1211,TBPM-PI,LJC 11036 | Smiles | CC1C2C(C(=O)N2C(=C1SC3CN(C3)C4=NCCS4)C(=O)OCOC(=O)C(C)(C)C)C(C)O | ||
Mechanism of Action
| In vitro |
Tebipenem Pivoxil has high intestinal apical membrane permeability due to plural intestinal transport routes, including the uptake transporters such as OATP1A2 and OATP2B1 as well as simple diffusion. This compound is quickly converted to tebipenem (TBPM), an active form. It is absorbed quickly, and the bioavailability is 71.4%, 59.1%, 34.8% and 44.9%, respectively, in mouse, rat, dog and monkey. Tebipenem shows the strongest bactericidal activity as early as 2 h after exposure at two times the MIC. It shows higher affinities for PBP 1A and PBP 2B, high-molecular-weight enzymes, and for PBP 3, a low-molecular-weight enzyme, than for PBP 2X. This chemical has a potent activity against Neisseria gonorrhoeae; its activity is comparable to it of cefixime that has most potent activity among oral antibiotics. |
|---|---|
| In vivo |
Tebipenem Pivoxil results in survival rate of 83%, compared with 25% survival for Amoxicillin and 0% survival for controls in animal model of otitis media. This compound exhibits slow tight-binding inhibition at low micromolar concentrations versus the chromogenic substrate nitrocefin. It acyl-enzyme complex remains stable for greater than 90 min and exists as mixture of the covalently bound drug and the bound retro-aldol cleavage product. |
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT04919954 | Completed | Diabetes|Wound Infection|ABSSSI|Healthy Volunteers |
Hartford Hospital|Spero Therapeutics |
July 1 2021 | Phase 1 |
| NCT04625855 | Completed | Healthy Volunteers |
Spero Therapeutics|Covance |
September 30 2020 | Phase 1 |
| NCT04376554 | Completed | Healthy Volunteers |
Spero Therapeutics|Iqvia Pty Ltd |
February 10 2020 | Phase 1 |
| NCT04178577 | Completed | Renal Impairment |
Spero Therapeutics |
December 6 2019 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































