research use only
Rivastigmine AChR inhibitor
Cat.No.S4687
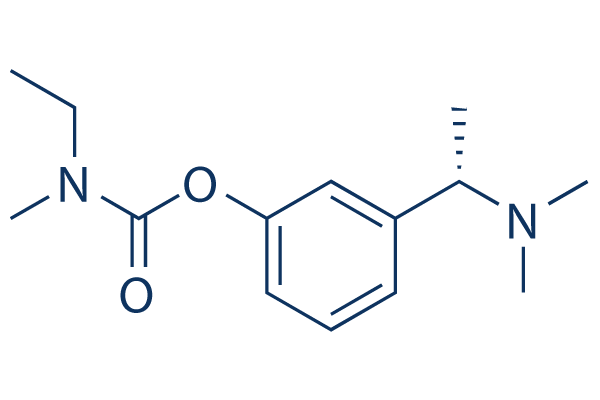
Chemical Structure
Molecular Weight: 250.34
Quality Control
Solubility
|
In vitro |
|
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 250.34 | Formula | C14H22N2O2 |
Storage (From the date of receipt) | 2 years -20°C liquid |
|---|---|---|---|---|---|
| CAS No. | 123441-03-2 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | SDZ-ENA 713, Exelon | Smiles | CCN(C)C(=O)OC1=CC=CC(=C1)C(C)N(C)C | ||
Mechanism of Action
| Targets/IC50/Ki |
cholinesterase
|
|---|---|
| In vivo |
Preclinical biochemical studies indicates that rivastigmine has central nervous system selectivity over peripheral inhibition. It ameliorates memory impairment in rats with forebrain lesions. The drug is rapidly absorbed orally, with a bioavailability of 0.355 and low protein binding (40%). Its elimination halflife is less than 2 hours, and it is converted to an inactive metabolite at the site of action, bypassing hepatic metabolic pathways. Its disposition essentially is unaltered in patients with renal or hepatic impairment. It also has dosedependent effects on AChE inhibition.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05768126 | Recruiting | Depressive Disorder |
UMC Utrecht|ZonMw: The Netherlands Organisation for Health Research and Development|St. Antonius Hospital|Tergooi Hospital |
September 29 2021 | Phase 4 |
| NCT01585272 | Completed | Alzheimer''s Dementia |
Novartis Pharmaceuticals|Novartis |
August 2012 | Phase 4 |
| NCT01073319 | Completed | Methamphetamine Dependence|Substance Abuse|Methamphetamine Abuse |
Baylor College of Medicine|National Institute on Drug Abuse (NIDA) |
July 2009 | Phase 1 |
| NCT01312363 | Completed | Alzheimer''s Disease |
Seoul National University Hospital|Seoul National University Bundang Hospital|Seoul St. Mary''s Hospital|Korea University|Bobath Memorial Hospital|Chung-Ang University Hosptial Chung-Ang University College of Medicine|Hanyang University|Kwandong University Myongji Hospital|Inha University Hospital|SungAe General Hospital|Ewha Womans University|Inje University|Hanyang University Seoul Hospital|Kyunghee University Medical Center|Hallym University Medical Center|Pusan National University Hospital|Konyang University Hospital|Wonkwang University Sanbon Medical Center|Yong-in Hyoja Geriatric Hospital|Jeju National University|Dong-A University Hospital |
June 2009 | -- |
| NCT00624663 | Completed | Rivastigmine Toxicity |
Tel-Aviv Sourasky Medical Center|Medical Corps Israel Defense Force |
January 2009 | Not Applicable |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































