research use only
Lenvatinib Mesylate VEGFR inhibitor
Cat.No.S5240
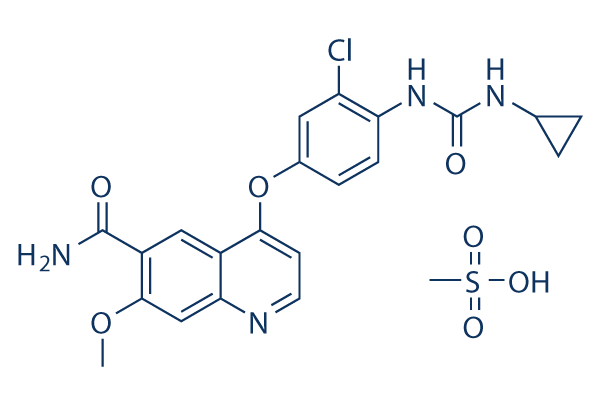
Chemical Structure
Molecular Weight: 522.96
Quality Control
| Related Targets | EGFR PDGFR FGFR c-Met Src MEK CSF-1R FLT3 HER2 c-Kit |
|---|---|
| Other VEGFR Inhibitors | SAR131675 SU 5402 Cediranib (AZD2171) Vatalanib (PTK787) 2HCl Anlotinib (AL3818) Dihydrochloride Linifanib (ABT-869) Apatinib (YN968D1) Apatinib (YN968D1) mesylate Semaxanib (SU5416) Ki8751 |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| DAOY | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for DAOY cells | 29435139 | |||
| SJ-GBM2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SJ-GBM2 cells | 29435139 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 29435139 | |||
| BT-37 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-37 cells | 29435139 | |||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 29435139 | |||
| SK-N-SH | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-SH cells | 29435139 | |||
| NB1643 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB1643 cells | 29435139 | |||
| LAN-5 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for LAN-5 cells | 29435139 | |||
| BT-12 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-12 cells | 29435139 | |||
| Rh18 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh18 cells | 29435139 | |||
| RD | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for RD cells | 29435139 | |||
| Rh41 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh41 cells | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for SK-N-MC cells | 29435139 | |||
| TC32 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for TC32 cells | 29435139 | |||
| Rh41 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for Rh41 cells | 29435139 | |||
| Rh18 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for Rh18 cells | 29435139 | |||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 39 mg/mL
(74.57 mM)
|
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 522.96 | Formula | C21H19ClN4O4.CH4O3S |
Storage (From the date of receipt) | 3 years -20°C powder |
|---|---|---|---|---|---|
| CAS No. | 857890-39-2 | -- | Storage of Stock Solutions |
|
|
| Synonyms | E7080 mesylate | Smiles | COC1=CC2=NC=CC(=C2C=C1C(=O)N)OC3=CC(=C(C=C3)NC(=O)NC4CC4)Cl.CS(=O)(=O)O | ||
Mechanism of Action
| Targets/IC50/Ki |
c-RET
VEGFR2
(Cell-free assay) 4.0 nM
VEGFR3
(Cell-free assay) 5.2 nM
VEGFR1
(Cell-free assay) 22 nM
PDGFRβ
(Cell-free assay) 39 nM
FGFR1
(Cell-free assay) 46 nM
PDGFRα
(Cell-free assay) 51 nM
Kit
(Cell-free assay) 100 nM
|
|---|---|
| In vitro |
Lenvatinib Mesylate (E7080) is an orally active inhibitor of multiple receptor tyrosine kinases including VEGF, FGF and SCF receptors. |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
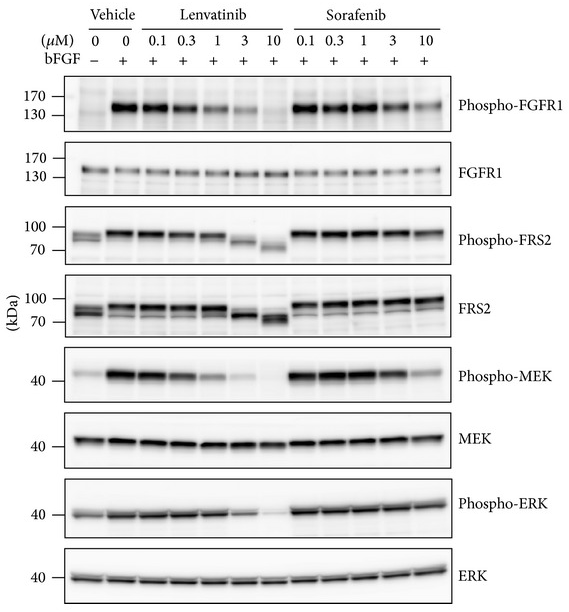
| Western blot | phospho-FGFR1 / FGFR1 /phospho-FRS2 / FRS2 / phospho-MEK / phospho-ERK phospho-RET / phospho-ERK Ki-67 / Cyclin D1 / CDK4 / p21 / p53 / Apaf-1 / p-NFκB / Bcl-2 / Cleaved-caspase 3 Vimentin / E-cadherin / Snail / Zeb1 |
|
25295214 |
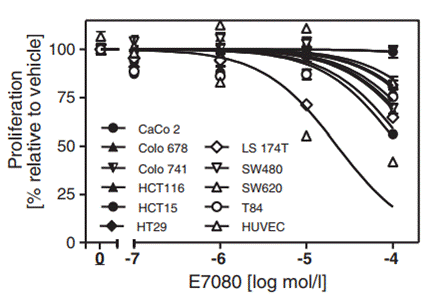
| Growth inhibition assay | Cell viability |
|
25425971 |
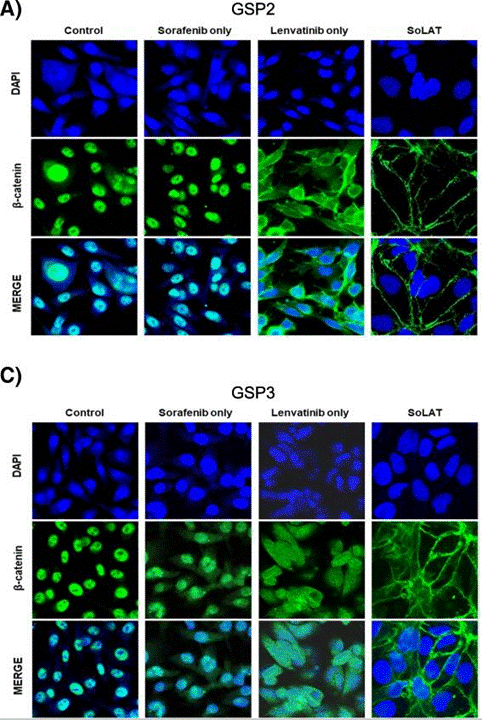
| Immunofluorescence | β-catenin |
|
30286728 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06161558 | Not yet recruiting | Neoplasms |
National Cancer Institute (NCI)|National Institutes of Health Clinical Center (CC) |
May 15 2024 | Phase 1 |
| NCT05846724 | Not yet recruiting | Kaposi Sarcoma|Classic Kaposi Sarcoma|Refractory Kaposi Sarcoma |
Fondazione IRCCS Ca'' Granda Ospedale Maggiore Policlinico |
February 1 2024 | Phase 2 |
| NCT05903833 | Not yet recruiting | Recurrent Vulvar Cancer|Persistent Vulvar Cancer|Metastatic Vulva Cancer|Locally Advanced Vulvar Cancer |
AGO Research GmbH |
January 1 2024 | Phase 2 |
| NCT05901194 | Not yet recruiting | Hepatocellular Carcinoma Non-resectable |
Assistance Publique - Hôpitaux de Paris|Laboratoire EISAI |
June 2023 | Phase 1|Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































