- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Tretazicar (CB1954) DNA alkylator chemical
Cat.No.S7829
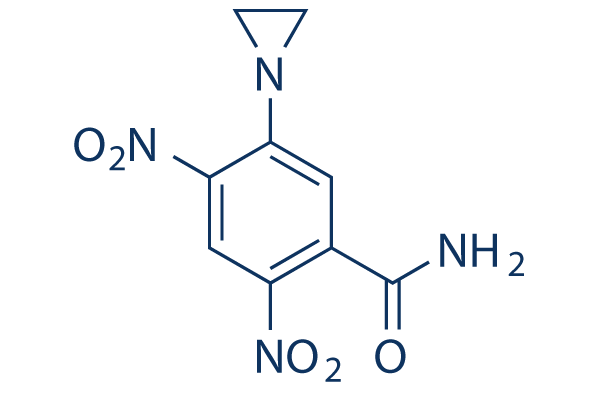
Chemical Structure
Molecular Weight: 252.18
Jump to
Quality Control
| Related Targets | HDAC PARP ATM/ATR DNA-PK WRN DNA/RNA Synthesis Topoisomerase PPAR Sirtuin Casein Kinase |
|---|---|
| Other DNA alkylator Products | Lomeguatrib Methyl methanesulfonate Lobaplatin (D-19466) Semustine Treosulfan |
Solubility
|
In vitro |
DMSO
: 50 mg/mL
(198.27 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 252.18 | Formula | C9H8N4O5 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 21919-05-1 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | NSC 115829 | Smiles | C1CN1C2=C(C=C(C(=C2)C(=O)N)[N+](=O)[O-])[N+](=O)[O-] | ||
Mechanism of Action
| In vitro |
The overexpression of nitroreductase oxidored nitro domain containing protein 1 (NOR1) is capable of converting the monofunctional alkylating agent Tretazicar (CB1954) into a toxic form by reducing its 4 nitro group, which is a potent cytotoxin. This compound enhances cell killing in the NPC cell line CNE1. The NOR1 gene enhances CB1954-mediated cell cytotoxicity through the upregulation of Grb2 expression and the activation of MAPK signal transduction in the HepG2 cell line.
|
|---|---|
| In vivo |
The Tretazicar (CB1954) system is used for specific ablation of cells in vivo. The effect of this inducible ablation system is dose-dependent. NTR-mediated cell killing by this compound does not require cell proliferation. The activated form cross-links DNA which presumably triggers the apoptosis cascade, resulting in rapid cell death. Specific and effective cell killing by NTR-CB1954 does not require a functional p53.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT04374240 | Completed | Prostate Cancer |
University of Birmingham|Janssen LP|Department of Health United Kingdom|Medical Research Council |
March 19 2013 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































