research use only
Doxazosin Adrenergic Receptor antagonist
Cat.No.S5782
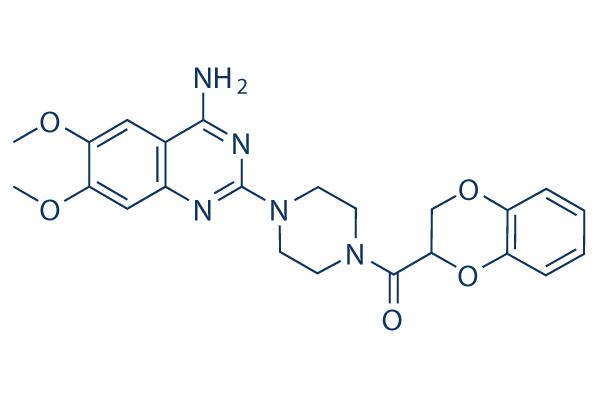
Chemical Structure
Molecular Weight: 451.48
Quality Control
| Related Targets | AChR 5-HT Receptor COX Calcium Channel Histamine Receptor Dopamine Receptor GABA Receptor TRP Channel Cholinesterase (ChE) GluR |
|---|---|
| Other Adrenergic Receptor Inhibitors | Zenidolol (ICI-118551) Hydrochloride L755507 Yohimbine HCl Atipamezole Higenamine hydrochloride Detomidine HCl Naftopidil Demethyl-Coclaurine Buflomedil HCl Fenoterol hydrobromide |
Solubility
|
In vitro |
DMSO
: 31 mg/mL
(68.66 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 451.48 | Formula | C23H25N5O5 |
Storage (From the date of receipt) | 3 years -20°C powder |
|---|---|---|---|---|---|
| CAS No. | 74191-85-8 | -- | Storage of Stock Solutions |
|
|
| Synonyms | UK 33274 | Smiles | COC1=C(C=C2C(=C1)C(=NC(=N2)N3CCN(CC3)C(=O)C4COC5=CC=CC=C5O4)N)OC | ||
Mechanism of Action
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT01946035 | Completed | Allergic Rhinitis |
University of Dundee |
September 2013 | Phase 4 |
| NCT01379898 | Completed | Pheochromocytoma |
University Medical Center Groningen|Radboud University Medical Center|UMC Utrecht|VU University of Amsterdam|Academisch Medisch Centrum - Universiteit van Amsterdam (AMC-UvA)|Leiden University Medical Center|Erasmus Medical Center|Maastricht University Medical Center|St. Antonius Hospital|Medisch Spectrum Twente|Maxima Medical Center|Canisius-Wilhelmina Hospital|Onze Lieve Vrouwe Gasthuis|Atrium Medical Center|Isala |
December 2011 | Phase 4 |
| NCT01062945 | Completed | Cocaine Addiction|Cocaine Abuse|Cocaine Dependence|Substance Abuse |
Baylor College of Medicine|National Institute on Drug Abuse (NIDA) |
January 2010 | Phase 1 |
| NCT00646841 | Completed | Hypertension |
Pfizer''s Upjohn has merged with Mylan to form Viatris Inc.|Pfizer |
February 2003 | Phase 4 |
| NCT00645034 | Completed | Benign Prostatic Hyperplasia |
Pfizer''s Upjohn has merged with Mylan to form Viatris Inc.|Pfizer |
November 2002 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































