research use only
Bifonazole Fungal inhibitor
Cat.No.S1854
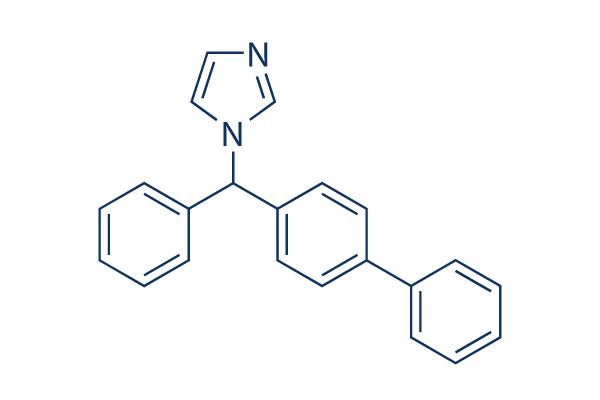
Chemical Structure
Molecular Weight: 310.39
Quality Control
| Related Targets | Integrase Bacterial Antibiotics Anti-infection Antiviral COVID-19 Parasite Reverse Transcriptase HIV HCV Protease |
|---|---|
| Other Fungal Inhibitors | Cycloheximide Tolnaftate Manogepix (E1210) Neticonazole Amorolfine HCl Thimerosal Isavuconazole Allicin Neticonazole Hydrochloride Juglone |
Solubility
|
In vitro |
DMSO
: 62 mg/mL
(199.74 mM)
Ethanol : 20 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 310.39 | Formula | C22H18N2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 60628-96-8 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | Bay h 4502,(±)-bifonazole | Smiles | C1=CC=C(C=C1)C2=CC=C(C=C2)C(C3=CC=CC=C3)N4C=CN=C4 | ||
Mechanism of Action
| In vitro |
Bifonazole, a broad spectrum antifungal agent, inhibits monooxygenase activity and induces a type II binding spectrum in 2B4dH(H226Y), a modified enzyme previously crystallized in the presence of 4-(4-chlorophenyl)imidazole (CPI). This compound (40 mM) releases Ca2+ from the store sensitive to 1 mM thapsigargin, an endopolasmic reticulum Ca2+ pump inhibitor. It per se induces capacitative Ca2+ entry while reduces 1 mM thapsigargin-induced capacitative Ca2+ entry. This chemical is calmodulin antagonists which most effectively reduce glycolysis and ATP level in B16 melanoma cells. It acts through allosteric regulation and detachment of glycolytic enzymes from cytoskeleton. This agent blocks PGE2 formation induced by 2 μm and 4 μm arachidonic acid clearly better than at 6 or 10 μm of the agonist. It shows the same characteristics in MC3T3-E1 and UMR-106 cells stimulated by ionomycin or various concentrations of arachidonic acid. |
|---|---|
| In vivo |
Bifonazole, but not clotrimazole, exhibits the characteristics of a peroxisome proliferator including hepatomegaly (increase in liver:body weight ratio), up to a 4-fold induction of lauric acid omega-hydroxylase activity and an 8-fold induction of palmitoyl-CoA oxidation by rat liver peroxisomes. This compound also induces P402B1/2B2, P4503A and P4501A1, but not P4502E1. |
References |
|
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































