|
How to Cite 1. For In-Text Citation (Materials & Methods): 2. For Key Resources Table: |
||
|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. We strive to reply to |
Technical Data
| Formula | C22H18N2 |
||||||
| Molecular Weight | 310.39 | CAS No. | 60628-96-8 | ||||
| Solubility (25°C)* | In vitro | DMSO | 62 mg/mL (199.74 mM) | ||||
| Ethanol | 20 mg/mL (64.43 mM) | ||||||
| Water | Insoluble | ||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||
Preparing Stock Solutions
Biological Activity
| Description | Bifonazole (Bay h 4502,(±)-bifonazole) is a substituted imidazole antifungal agent. |
|---|---|
| In vitro | Bifonazole, a broad spectrum antifungal agent, inhibits monooxygenase activity and induces a type II binding spectrum in 2B4dH(H226Y), a modified enzyme previously crystallized in the presence of 4-(4-chlorophenyl)imidazole (CPI). This compound (40 mM) releases Ca2+ from the store sensitive to 1 mM thapsigargin, an endopolasmic reticulum Ca2+ pump inhibitor. It per se induces capacitative Ca2+ entry while reduces 1 mM thapsigargin-induced capacitative Ca2+ entry. This chemical is calmodulin antagonists which most effectively reduce glycolysis and ATP level in B16 melanoma cells. It acts through allosteric regulation and detachment of glycolytic enzymes from cytoskeleton. This agent blocks PGE2 formation induced by 2 μm and 4 μm arachidonic acid clearly better than at 6 or 10 μm of the agonist. It shows the same characteristics in MC3T3-E1 and UMR-106 cells stimulated by ionomycin or various concentrations of arachidonic acid. |
| In vivo | Bifonazole, but not clotrimazole, exhibits the characteristics of a peroxisome proliferator including hepatomegaly (increase in liver:body weight ratio), up to a 4-fold induction of lauric acid omega-hydroxylase activity and an 8-fold induction of palmitoyl-CoA oxidation by rat liver peroxisomes. This compound also induces P402B1/2B2, P4503A and P4501A1, but not P4502E1. |
Protocol (from reference)
References
|
Customer Product Validation
-
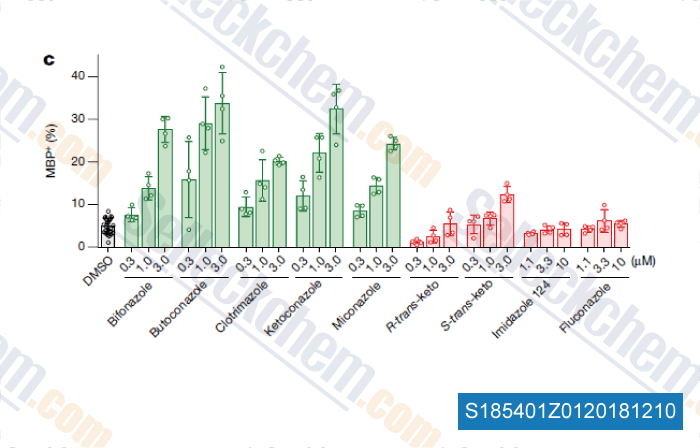
Data from [ , , Nature, 2018, 560(7718):372-376 ]
Selleck's Bifonazole Has Been Cited by 1 Publication
| Accumulation of 8,9-unsaturated sterols drives oligodendrocyte formation and remyelination [Hubler Z, et al. Nature, 2018, 560(7718):372-376] | PubMed: 30046109 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
