research use only
Tigecycline Antineoplastic and Immunosuppressive Antibiotics inhibitor
Cat.No.S1403
Chemical Structure
Molecular Weight: 585.65
Quality Control
| Related Targets | Integrase Bacterial Antibiotics Anti-infection Fungal Antiviral COVID-19 Parasite Reverse Transcriptase HIV |
|---|---|
| Other Antineoplastic and Immunosuppressive Antibiotics Inhibitors | Staurosporine (STS) Cyclosporin A Oligomycin A (MCH 32) Puromycin Dihydrochloride Nigericin sodium salt Geldanamycin (NSC 122750) Honokiol Streptozotocin (STZ) Sodium Monensin (NSC 343257) Cephalomannine |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| THP-1 | Antibacterial assay | 24 hrs | Antibacterial activity against Staphylococcus aureus SCV isolated from cystic fibrosis patient infected in human THP-1 cells assessed as log reduction of intracellular CFU level after 24 hrs in presence of thymidine | 19188393 | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(170.75 mM)
Water : 100 mg/mL Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 585.65 | Formula | C29H39N5O8 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 220620-09-7 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | GAR-936, WAY-GAR-936, TBG-MINO | Smiles | CC(C)(C)NCC(=O)NC1=CC(=C2CC3CC4C(C(=O)C(=C(C4(C(=O)C3=C(C2=C1O)O)O)O)C(=O)N)N(C)C)N(C)C | ||
Mechanism of Action
| In vitro |
Tigecycline evades the Tet(A-E) efflux pumps, which account for most acquired resistance to tetracycline and minocycline in Enterobacteriaceae and Acinetobacter spp. This compound binds to bacterial ribosomes that have been modified by the Tet(M) protein, a mechanism that compromises all available tetracyclines, and which is frequent in Gram-positive cocci and Neisseria spp. It remains vulnerable to the chromosomally-encoded multidrug efflux pumps of Proteeae and Pseudomonas aeruginosa, and to Tet(X), a tetracycline-degrading mono-oxygenase found, albeit rarely, in Bacteroides spp. Its MICs for enterococci, staphylococci, and streptococci are mostly 0.06–0.25 mg/L, again with little or no skew to the distribution. This agent is prone to oxidation, and MIC values, particularly for the most susceptible isolates, may be raised if the drug is added to broth that has become oxygenated during storage, or if drug-containing media are stored before inoculation. It is a poor substrate for tetracycline-specific efflux pumps, and it still attaches to ribosomes that have been modified by the Tet(M) protein. This antibacterial has demonstrated activity against a wide variety of gram-positive and gram-negative pathogens, including multidrug-resistant strains. It is active against many gram-positive and -negative organisms, including methicillin-resistantStaphylococcus aureus, vancomycin-intermediate and -resistant enterococci, and extended-spectrum β-lactamase–producing Escherichia coli and Klebsiella pneumoniae. This compound exhibits antibacterial activity against a wide spectrum of aerobic and anaerobic bacteria. It is a broad-spectrum, protein-inhibiting, antibacterial agent possessing activity against strains resistant to other chemotherapeutic agents. This chemical demonstrates in vitro activities against the GISA and the methicillin-resistant and methicillin-susceptible staphylococcal strains tested (MICs at which 90% of isolates tested are inhibited [MIC90s], 0.5 to 1 μg/ml). It has MIC90s of 0.25 μg/ml for all of the S. pneumoniae strains and demonstrates similar activities against all of the S. pneumoniae strains tested. |
|---|---|
| In vivo |
Tigecycline is bactericidal against methicillin-susceptible S. aureus (MSSA) in the rabbit osteomyelitis model and exhibits good, but not excellent, activity in Legionellapneumophila pneumonia in guinea pigs. This compound at 50 mg/kg twice daily was not toxic to mice. It is effective in inhibiting NSCLC growth in vivo through decreasing proliferation and increasing apoptosis of tumor cells. |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | E-cadherin / Vimentin CDK2 / Cyclin E Cox-1 / Cox-2 / Cox-4 p-AMPKα / AMPKα / p-mTOR / mTOR / p-p70S6K / p70S6K / p-4E-BP-1 / 4E-BP1 p62 / LC3-I / LC3-II Cyclin D1 / CDK2 / p21 |
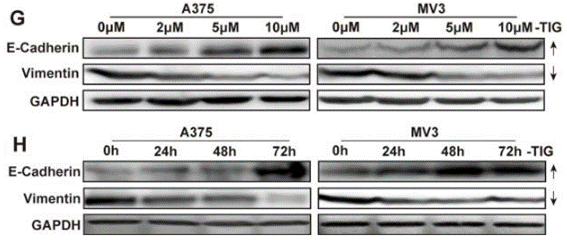
|
26621850 |
| Growth inhibition assay | Cell viability |
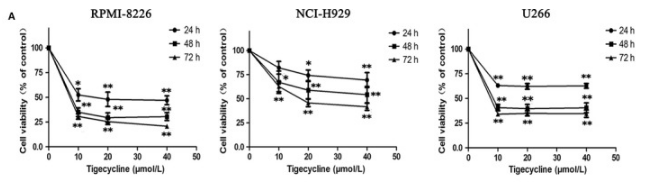
|
30247801 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06049771 | Recruiting | Carbapenem-resistant Enterobacteriaceae |
Phramongkutklao College of Medicine and Hospital|Silpakorn University |
September 17 2023 | Not Applicable |
| NCT05698160 | Not yet recruiting | Blood Coagulation Disorder |
The Affiliated Nanjing Drum Tower Hospital of Nanjing University Medical School |
May 1 2023 | -- |
| NCT04937894 | Recruiting | Infectious Disease |
Shandong University|Shandong Provincial Hospital |
June 1 2021 | -- |
| NCT04724798 | Unknown status | Extracorporeal Membrane Oxygenation|Pharmacokinetics|Tigecycline |
Nanfang Hospital Southern Medical University |
January 20 2020 | -- |
| NCT04489459 | Unknown status | Treatment of Blood Stream Infections Due to Multidrug-Resistant Klebsiella Pneumoniae |
Al-Azhar University |
September 21 2019 | Phase 4 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































