research use only
Silodosin Adrenergic Receptor antagonist
Cat.No.S1613
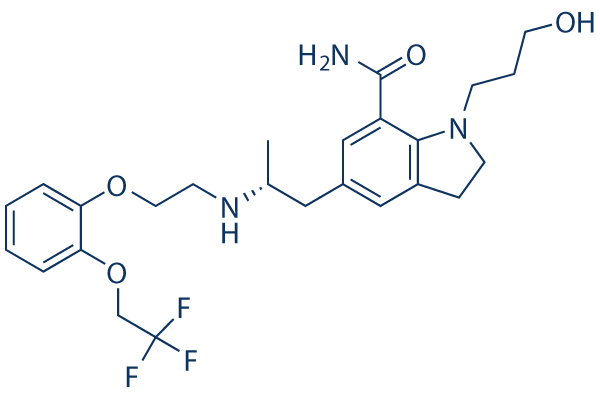
Chemical Structure
Molecular Weight: 495.53
Quality Control
Solubility
|
In vitro |
DMSO
: 99 mg/mL
(199.78 mM)
Ethanol : 99 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 495.53 | Formula | C25H32F3N3O4 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 160970-54-7 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | KAD 3213, KMD 3213 | Smiles | CC(CC1=CC2=C(C(=C1)C(=O)N)N(CC2)CCCO)NCCOC3=CC=CC=C3OCC(F)(F)F | ||
Mechanism of Action
| Targets/IC50/Ki |
α1-adrenergic receptor
|
|---|---|
| In vitro |
Silodosin shows higher selectivity for the alpha(1A)-AR subtype than tamsulosin hydrochloride, naftopidil or prazosin hydrochloride (affinity is highest for tamsulosin hydrochloride, followed by this compound, prazosin hydrochloride and naftopidil in that order). This compound and tadalafil have synergistic inhibitor effects on nerve-mediated contractions of human and rat isolated prostates in rats.
|
| In vivo |
Silodosin strongly antagonizes noradrenaline-induced contractions in rabbit lower urinary tract tissues (including prostate, urethra and bladder trigone, with pA(2) or pKb values of 9.60, 8.71 and 9.35, respectively). This compound significantly inhibits the phenylephrine-induced increase in intraurethral pressure (versus the vehicle-treated group) at 12 hours, 18 hours, and 24 hours after its oral administration in rats. It (0.1-0.3 mg/kg) or prazosin (0.03-0.1 mg/kg) reduces obstruction-induced increases in intraluminal ureter pressures by 21-37% or 18-40% respectively. This chemical inhibits contractions of the rat and human isolated ureters and has excellent functional selectivity in vivo to relieve pressure-load of the rat obstructed ureter. It (0.3-300 mg/kg) dose-dependently inhibits the hypogastric nerve stimulation-induced increase in intraurethral pressure (without significant hypotensive effects) in both young and old dogs with benign prostatic hyperplasia.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT01757769 | Completed | Benign Prostatic Hyperplasia |
RECORDATI GROUP |
May 2011 | Phase 4 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































