research use only
Febuxostat ROS inhibitor
Cat.No.S1547
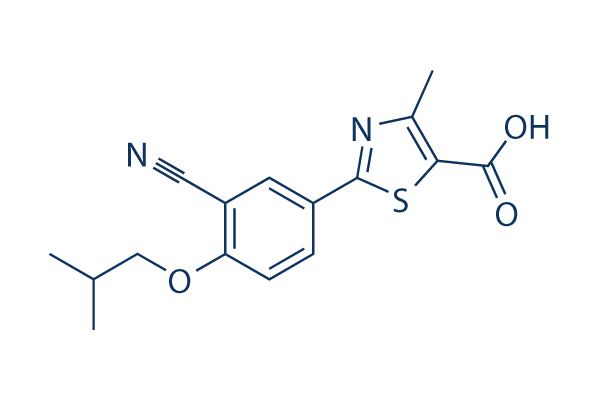
Chemical Structure
Molecular Weight: 316.37
Quality Control
Solubility
|
In vitro |
DMSO
: 63 mg/mL
(199.13 mM)
Ethanol : 63 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 316.37 | Formula | C16H16N2O3S |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 144060-53-7 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | TMX 67, TEI-6720 | Smiles | CC1=C(SC(=N1)C2=CC(=C(C=C2)OCC(C)C)C#N)C(=O)O | ||
Mechanism of Action
| Targets/IC50/Ki |
xanthine oxidase
0.6 nM(Ki)
|
|---|---|
| In vitro |
Febuxostat displays potent mixed-type inhibition of the activity of purified bovine milk xanthine oxidase, with Ki and Ki' values of 0.6 nM and 3.1 nM respectively, indicating inhibition of both the oxidized and reduced forms of xanthine oxidase.
|
| In vivo |
Febuxostat (5–6 mg/kg/day) combined with fructose significantly lowers blood pressure, UA, triglycerides, and insulin in rats compared with fructose alone. This compound (5–6 mg/kg/day) combined with fructose also reduces glomerular pressure, renal vasoconstriction, and afferent arteriolar area in rats compared with fructose alone. It prevents hyperuricemia in 5/6 nephrectomy (5/6 Nx)+oxonic acid (OA)+Febuxostat(Fx) rats and ameliorates proteinuria, preserves renal function and prevents glomerular hypertension in both 5/6 nephrectomy (5/6 Nx)+vehicle (V)+Febuxostat(Fx) and 5/6 nephrectomy (5/6 Nx)+oxonic acid (OA)+Febuxostat(Fx) groups. This chemical (5 mg/kg/d by gavage for 8 days) treatment after transverse aortic constriction (TAC) attenuates the TAC-induced left ventricular (LV) hypertrophy and dysfunction. It blunts the TAC-induced increases in nitrotyrosine (indicating reduced myocardial oxidative stress), p-Erk(Thr202/Tyr204), and p-mTOR(Ser2488), with no effect on total Erk or total mTOR. This agent significantly suppresses oxonic acid activity, and thereby reduces oxidative stress in Sprague-Dawley rats with right nephrectomy and left renal I/R injury, as assessed by nitrotyrosine, thiobarbituric acid-reactive substances (TBARS) and urine 8-isoprostane. It also reduces the induction of endoplasmic reticulum (ER) stress in Sprague-Dawley rats with right nephrectomy and left renal I/R injury, as assessed by GRP-78, ATF4, and CHOP.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05109936 | Not yet recruiting | Gout |
University Hospital Rouen |
January 1 2022 | Phase 3 |
| NCT04853160 | Completed | Gout|Cardiovascular Diseases |
Takeda |
August 15 2020 | -- |
| NCT04157959 | Unknown status | Hyperuricemia |
Jiangsu HengRui Medicine Co. Ltd. |
October 14 2019 | Phase 1 |
| NCT03918551 | Completed | Bioequivalence |
Pharmtechnology LLC|Altasciences Company Inc. |
May 3 2019 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































