research use only
Ethionamide Antibiotics chemical
Cat.No.S1777
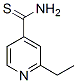
Chemical Structure
Molecular Weight: 166.24
Quality Control
| Related Targets | Integrase Bacterial Anti-infection Fungal Antiviral COVID-19 Parasite Reverse Transcriptase HIV HCV Protease |
|---|---|
| Other Antibiotics Inhibitors | G418 Sulfate (Geneticin) Nanchangmycin Fusidine Sitafloxacin Hydrate Gamithromycin Tildipirosin Spiramycin Nadifloxacin 6-Aminopenicillanic acid Thiamphenicol |
Solubility
|
In vitro |
DMSO
: 33 mg/mL
(198.5 mM)
Ethanol : 17 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 166.24 | Formula | C8H10N2S |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 536-33-4 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | Bayer 5312 | Smiles | CCC1=NC=CC(=C1)C(=S)N | ||
Mechanism of Action
| In vitro |
Ethionamide is a structural analogue of isoniazid (INH), both are pro-drugs that need to be activated by mycobacterial enzymes to exert their antimicrobial activity. Its mechanism of action is thought to be identical to INH although the pathway of activation is distinct from that of INH. This compound is activated by an EthA enzyme, leading to the formation of a Soxide metabolite that has considerably better activity than the parent drug. It inhibits both biofilm formation and viability of mature biofilms. This chemical reduces the content of ergosterol in Cryptococcus spp. planktonic cells and destabilized or permeabilized the fungal cell membrane, leading to leakage of macromolecules. It is in general toxic at concentrations above 0.50 mM to HepG2, Caco-2, and RAW macrophage cells, but the toxicity is drastically reduced when this compound is loaded into the microparticles. It shows a fast metabolization process in the presence of the thermally carbonized- Porous silicon (TCPSi) particles. It is activated in Mycobacterium tuberculosis by the protein encoded by the gene Rv3854c. This compound appear to disrupt cell wall biosynthesis and have at least one common cellular target, the enoyl-acyl carrier protein reductase InhA.
|
References |
|
|---|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05258877 | Completed | Tuberculosis Pulmonary |
BioVersys AG|TASK Applied Science |
June 10 2022 | -- |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































