Archival Context
This article serves as a digital record of the pivotal CSRC/FDA Workshop held in 2016. As cited in Therapeutic Innovation & Regulatory Science, this meeting focused on evolving regulatory paradigms for assessing the proarrhythmic risk of New Chemical Entities (NCEs), with a specific emphasis on leveraging early Phase I ECG data to replace late-stage Thorough QT (TQT) studies.
Executive Summary
The assessment of cardiac safety is a critical gatekeeper in the development of New Chemical Entities (NCEs). For years, the industry operated under the ICH E14 guideline, necessitating a dedicated “Thorough QT” (TQT) study in late-stage development. While effective, this approach is resource-intensive and often occurs too late to influence molecule design.
The 2016 CSRC/FDA Workshop marked a turning point. Regulators and industry leaders gathered to discuss a new paradigm: integrating robust pre-clinical screening with early Phase I ECG assessment. This shift allows developers to characterize proarrhythmic risk earlier, faster, and more mechanistically. Safety is no longer just a clinical concern—it is a chemical design parameter.
Part I: The Shift to Early Phase I ECG Assessment
The primary focus of the workshop was evaluating whether intensive ECG monitoring during routine Phase I (First-in-Human) trials could effectively replace the standalone TQT study.
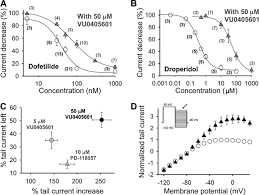
Concentration-QTc (C-QTc) Modeling
The workshop highlighted that C-QTc modeling in Phase I is often more sensitive than categorical analysis. By drawing high-precision ECGs at multiple timepoints matched with pharmacokinetic (PK) samples, researchers can construct a linear model of the drug’s effect on the QT interval.
The Role of Assay Sensitivity
To prove that a Phase I study is capable of detecting a QT effect, the FDA requires “Assay Sensitivity.” In traditional TQT studies, Moxifloxacin is used as a pharmacological positive control. However, giving it to healthy volunteers in complex Phase I designs is difficult. Therefore, regulators are increasingly accepting “High Confidence” negative results backed by strong pre-clinical ion channel data.
Part II: The Pre-Clinical Foundation – Screening New Chemical Entities
FDA speakers emphasized that clinical safety waivers are contingent upon a “low risk” pre-clinical profile. Before an NCE enters Phase I, it must undergo rigorous screening against cardiac ion channels: hERG ($I_{Kr}$), Cav1.2, and Nav1.5.
Chemical Liability: Modern workflows involve screening Diversity Compound Libraries to identify scaffolds that lack “structural alerts” while maintaining therapeutic potency.
Part III: The Role of Reference Standards in Validation
A recurring theme in regulatory science is “Validation.” Whether it is a cellular patch-clamp assay or a Stem Cell (hiPSC-CM) model, the system must be calibrated using Reference Compounds. The reliability of these studies depends entirely on the purity of the reference standards. Using an impure chemical probe can lead to false negatives, potentially allowing a dangerous NCE to proceed—a costly mistake the 2016 workshop sought to prevent.
Conclusion and Future Outlook
The 2016 CSRC/FDA Workshop codified the “Phase I ECG” pathway. For the pharmaceutical industry, this evolution underscores that safety starts at synthesis. By leveraging comprehensive compound screening libraries and rigorous in vitro models, developers can ensure their NCEs are robust enough to withstand the scrutiny of early clinical assessment.
References
- Strauss, D. G., et al. Comprehensive In Vitro Proarrhythmia Assay (CiPA) Update. Therapeutic Innovation & Regulatory Science, 2019.
- ICH E14/S7B Implementation Working Group. ICH E14/S7B Questions and Answers, 2022.
- Darpo, B., et al. The IQ-CSRC Prospective Study: Can Early Phase 1 ECG Data Replace the Thorough QT Study? Annals of Noninvasive Electrocardiology, 2014.