- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Sacituzumab-govitecan ADC Cytotoxin chemical
Cat.No.E2841
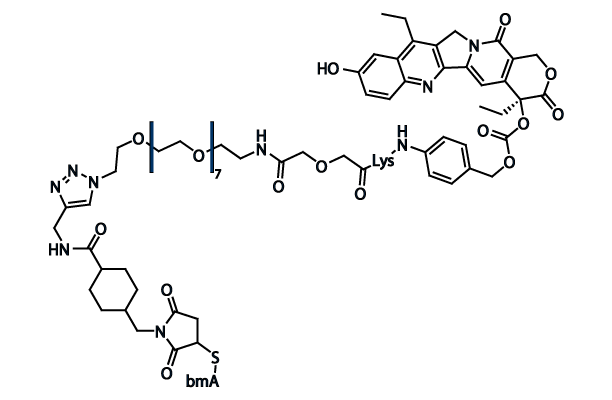
Chemical Structure
Molecular Weight: 160000
Jump to
Click to purchase products related to Sacituzumab govitecan
Quality Control
Solubility
|
In vitro |
|
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 160000 | Formulation | 10mg/mL in PBS | DAR | 7.6 |
|---|---|---|---|---|---|
| Antibody | Sacituzumab | ADC Cytotoxin | SN-38 | Formula | C76H104N12O24S |
| CAS No. | 1491917-83-9 (ADC) | Storage (From the date of receipt) | 1 year -80°C in solvent | Shipping | Room temperature shipping(Stability testing shows this product can be shipped without any cooling measures.) |
Mechanism of Action
| In vitro |
Sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC) made from a humanized anti-Trop-2 monoclonal antibody (hRS7) conjugated with the active metabolite of irinotecan, SN-38, shows anticancer activity in cancers expressing Trop-2, including gastric and pancreatic cancer. |
References |
|---|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06161532 | Not yet recruiting | Small Cell Carcinoma of the Bladder|Small Cell Carcinoma of the Urinary Tract|Squamous Cell Carcinoma of the Bladder|Squamous Cell Carcinoma of the Urinary Tract|Primary Adenocarcinoma of the Bladder|Primary Adenocarcinoma of the Urinary Tract|Renal Medullary Carcinoma|Squamous Cell Carcinoma of the Penis |
National Cancer Institute (NCI)|National Institutes of Health Clinical Center (CC) |
May 15 2024 | Phase 2 |
| NCT06236269 | Not yet recruiting | Breast Cancer Stage IV |
SOLTI Breast Cancer Research Group |
February 20 2024 | Phase 2 |
| NCT06028932 | Recruiting | Ovarian Carcinoma |
Yale University|Gilead Sciences |
January 8 2024 | Phase 2 |
| NCT06167317 | Recruiting | Advanced Solid Tumors |
Gilead Sciences |
January 9 2024 | Phase 1 |
| NCT05838521 | Recruiting | Cervical Cancer |
Yale University|Gilead Sciences |
June 2 2023 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
Frequently Asked Questions
Question 1:
What's the solvent of this ADC drug? And what's its concentration?
Answer:
The solvent is a PBS solution. Its concentration is 10mg/ml.
Question 2:
What is its molecular weight?
Answer:
It contains on average 7 to 8 molecules of SN-38 per antibody molecule. This compound has a molecular weight of approximately 160 kilodaltons. The molecular weight of the drug (SN-38) and linker other than the antibody is 1601.8.






































