research use only
Rimiducid (AP1903) Dimerizer Agent
Cat.No.S9726
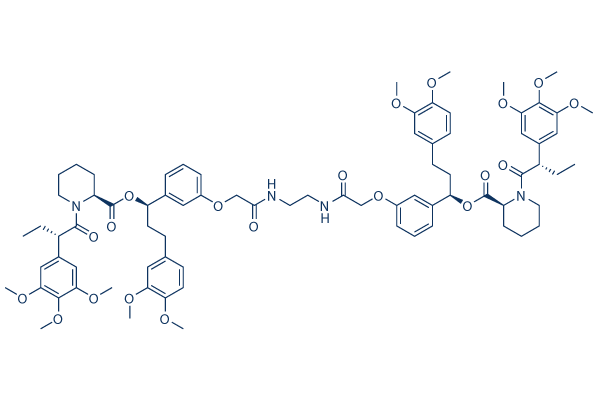
Chemical Structure
Molecular Weight: 1411.63
Quality Control
| Related Targets | Bcl-2 Caspase PD-1/PD-L1 Ferroptosis p53 Apoptosis related Synthetic Lethality STAT TNF-alpha Ras |
|---|---|
| Other FKBP Inhibitors | dTAG-13 Shield-1 (Shld1) AP20187 (B/B Homodimerizer) S107 hydrochloride dTAGV-1 TFA SAFit2 |
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(70.84 mM)
Ethanol : 100 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 1411.63 | Formula | C78H98N4O20 |
Storage (From the date of receipt) | 3 years -20°C powder |
|---|---|---|---|---|---|
| CAS No. | 195514-63-7 | -- | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | CCC(C(=O)N1CCCCC1C(=O)OC(CCC2=CC(=C(OC)C=C2)OC)C3=CC(=CC=C3)OCC(=O)NCCNC(=O)COC4=CC=CC(=C4)C(CCC5=CC(=C(OC)C=C5)OC)OC(=O)C6CCCCN6C(=O)C(CC)C7=CC(=C(OC)C(=C7)OC)OC)C8=CC(=C(OC)C(=C8)OC)OC | ||
Mechanism of Action
| Targets/IC50/Ki |
FKBP fusion protein
|
|---|---|
| In vitro |
Rimiducid (AP1903) induces effective and dose-dependent apoptosis in the culture expressing dimer-dependent Fas constructors, indicating that it causes the activation of Fas signaling without interference from endogenous FKBP. |
| In vivo |
Eliciting a dose-dependent decrease in serum hGH levels with a half-maximal effective dose of 0.4±0.1 mg/kg, Rimiducid (AP1903) is functional in activating Fas signaling in vivo. |
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05298995 | Recruiting | Brain Tumor Pediatric|Medulloblastoma Childhood|Embryonal Tumor|High Grade Glioma|Diffuse Midline Glioma|Diffuse Intrinsic Pontine Glioma|Brain Tumor Adult |
Bambino Gesù Hospital and Research Institute |
November 9 2023 | Phase 1 |
| NCT03807063 | Withdrawn | Acute Bilineal Leukemia|Myelodysplastic/Myeloproliferative Neoplasm|Myelodysplastic/Myeloproliferative Neoplasm Unclassifiable|Myeloproliferative Neoplasm|Recurrent Acute Biphenotypic Leukemia|Recurrent Acute Lymphoblastic Leukemia|Recurrent Acute Myeloid Leukemia|Recurrent Blastic Plasmacytoid Dendritic Cell Neoplasm|Recurrent Chronic Lymphocytic Leukemia|Recurrent Chronic Myelogenous Leukemia BCR-ABL1 Positive|Recurrent Chronic Myelomonocytic Leukemia|Recurrent Myelodysplastic Syndrome |
Fred Hutchinson Cancer Center|National Cancer Institute (NCI)|Bellicum Pharmaceuticals |
January 2 2020 | Phase 1 |
| NCT02849886 | Unknown status | Graft Versus Host Disease|Hematological Malignancies |
Centre Hospitalier Universitaire de Besancon|Etablissement Français du Sang|Bellicum Pharmaceuticals |
April 10 2019 | Phase 1|Phase 2 |
| NCT03741127 | Active not recruiting | Multiple Myeloma |
Poseida Therapeutics Inc.|California Institute for Regenerative Medicine (CIRM) |
October 29 2018 | Phase 1 |
| NCT02477878 | Active not recruiting | Leukemia|Myelodysplastic Syndromes|Lymphoma|Multiple Myeloma|Hematologic Neoplasms |
Bellicum Pharmaceuticals |
July 2016 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































