research use only
Nifuroxazide STAT inhibitor
Cat.No.S4182
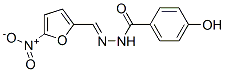
Chemical Structure
Molecular Weight: 275.22
Quality Control
| Related Targets | EGFR JAK Pim |
|---|---|
| Other STAT Inhibitors | Napabucasin (BBI608) Stattic NSC 74859 (S3I-201) Cryptotanshinone (Tanshinone C) C188-9 (TTI-101) SH-4-54 BP-1-102 AS1517499 HO-3867 Homoharringtonine (HHT) |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| human U3A cells | Function assay | 1 h | Inhibition of STAT3 expressed in human U3A cells after 1 hr by luciferase reporter gene assay, EC50=3 μM | |||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 55 mg/mL
(199.84 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 275.22 | Formula | C12H9N3O5 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 965-52-6 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | C1=CC(=CC=C1C(=O)NN=CC2=CC=C(O2)[N+](=O)[O-])O | ||
Mechanism of Action
| Targets/IC50/Ki |
STAT1
STAT3
STAT5
|
|---|---|
| In vitro |
Nifuroxazide is a nitrofuran compound inhibitor of STAT transcription factor signaling. This compound is described to block constitutive phosphorylation of STAT3 by reducing Jak kinase autophosphorylation, decreasing the viability of myeloma cells depending on constitutive STAT3 activity for survival while not affecting normal peripheral blood mononuclear cells. It produces decreases in tyrosine phosphorylation of Jak2 and Tyk2, and showed no effects on EGF receptor tyrosine kinase or Src kinase, indicating a relative specificity of this chemical for Jak2 and Tyk2. It shows no inhibition of Akt or MAPK phosphorylation. This inhibitor inhibits the constitutive phosphorylation of STAT3 in MM cells by reducing Jak kinase autophosphorylation, and leads to down-regulation of the STAT3 target gene Mcl-1. It causes a decrease in viability of primary myeloma cells and myeloma cell lines containing STAT3 activation, but not normal peripheral blood mononuclear cells. Although bone marrow stromal cells provide survival signals to myeloma cells, this compound can overcome this survival advantage. Reflecting the interaction of STAT3 with other cellular pathways, it shows enhanced cytotoxicity when combined with either the histone deacetylase inhibitor depsipeptide or the MEK inhibitor UO126.
|
References |
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Growth inhibition assay | Cell viability |
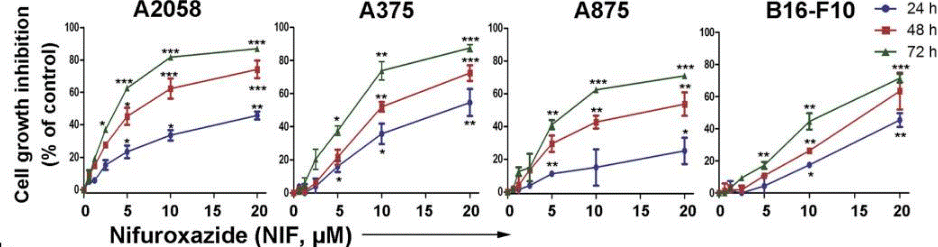
|
26830149 |
| Western blot | p-STAT3 / STAT3 / p-FAK / FAK / p-Src / Src MMP-9 / MMP-2 |
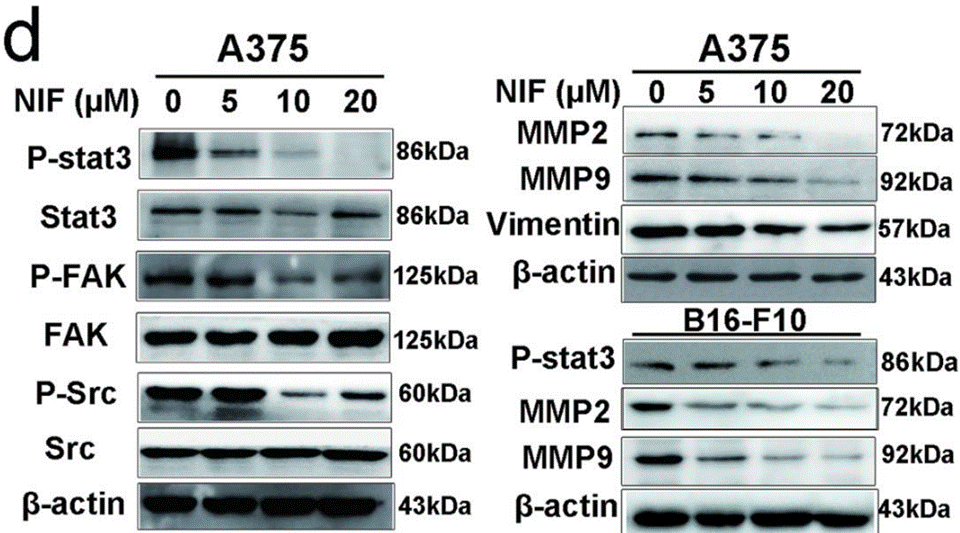
|
26830149 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05754996 | Recruiting | Hepatic Encephalopathy |
Cairo University |
March 12 2023 | Phase 3 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































