research use only
Lapatinib (GW572016) EGFR/HER2 Inhibitor
Cat.No.S2111
Chemical Structure
Molecular Weight: 581.06
Quality Control
| Related Targets | VEGFR PDGFR FGFR c-Met Src MEK CSF-1R FLT3 HER2 c-Kit |
|---|---|
| Other EGFR Inhibitors | Lazertinib (YH25448) Icotinib Hydrochloride Sunvozertinib AG-490 AG-1478 Canertinib (CI-1033) Rociletinib (CO-1686) WZ4002 Poziotinib (NOV120101, HM781-36B) Genistein |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| LB2241-RCC | Growth Inhibition Assay | IC50=1.15403 μM | SANGER | |||
| TE-9 | Growth Inhibition Assay | IC50=0.74453 μM | SANGER | |||
| A388 | Growth Inhibition Assay | IC50=0.72258 μM | SANGER | |||
| TE-12 | Growth Inhibition Assay | IC50=0.49057 μM | SANGER | |||
| EKVX | Growth Inhibition Assay | IC50=0.44874 μM | SANGER | |||
| BB30-HNC | Growth Inhibition Assay | IC50=0.24654 μM | SANGER | |||
| BT-474 | Growth Inhibition Assay | IC50=0.21315 μM | SANGER | |||
| DSH1 | Growth Inhibition Assay | IC50=0.09396 μM | SANGER | |||
| ECC12 | Growth Inhibition Assay | IC50=0.09231 μM | SANGER | |||
| OCUB-M | Growth Inhibition Assay | IC50=0.0574 μM | SANGER | |||
| HCC2218 | Growth Inhibition Assay | IC50=0.05326 μM | SANGER | |||
| NCI-H1648 | Growth Inhibition Assay | IC50=0.02544 μM | SANGER | |||
| LB996-RCC | Growth Inhibition Assay | IC50=1.36228 μM | SANGER | |||
| LC-1F | Growth Inhibition Assay | IC50=1.38244 μM | SANGER | |||
| TE-6 | Growth Inhibition Assay | IC50=1.55201 μM | SANGER | |||
| A253 | Growth Inhibition Assay | IC50=1.97335 μM | SANGER | |||
| OS-RC-2 | Growth Inhibition Assay | IC50=1.99199 μM | SANGER | |||
| TE-1 | Growth Inhibition Assay | IC50=2.0483 μM | SANGER | |||
| RL95-2 | Growth Inhibition Assay | IC50=3.1567 μM | SANGER | |||
| LS-513 | Growth Inhibition Assay | IC50=3.40041 μM | SANGER | |||
| DJM-1 | Growth Inhibition Assay | IC50=3.46975 μM | SANGER | |||
| NMC-G1 | Growth Inhibition Assay | IC50=3.54501 μM | SANGER | |||
| TE-10 | Growth Inhibition Assay | IC50=3.55356 μM | SANGER | |||
| TE-5 | Growth Inhibition Assay | IC50=4.0373 μM | SANGER | |||
| TK10 | Growth Inhibition Assay | IC50=4.16522 μM | SANGER | |||
| UACC-812 | Growth Inhibition Assay | IC50=4.56153 μM | SANGER | |||
| SW962 | Growth Inhibition Assay | IC50=5.02159 μM | SANGER | |||
| SW954 | Growth Inhibition Assay | IC50=5.39245 μM | SANGER | |||
| COLO-668 | Growth Inhibition Assay | IC50=5.72667 μM | SANGER | |||
| LB1047-RCC | Growth Inhibition Assay | IC50=5.80046 μM | SANGER | |||
| NB5 | Growth Inhibition Assay | IC50=6.21001 μM | SANGER | |||
| NTERA-S-cl-D1 | Growth Inhibition Assay | IC50=6.26561 μM | SANGER | |||
| IST-MEL1 | Growth Inhibition Assay | IC50=6.43694 μM | SANGER | |||
| GI-1 | Growth Inhibition Assay | IC50=6.51682 μM | SANGER | |||
| TGBC1TKB | Growth Inhibition Assay | IC50=7.07183 μM | SANGER | |||
| GT3TKB | Growth Inhibition Assay | IC50=7.22744 μM | SANGER | |||
| EVSA-T | Growth Inhibition Assay | IC50=7.42811 μM | SANGER | |||
| D-502MG | Growth Inhibition Assay | IC50=7.48894 μM | SANGER | |||
| TE-8 | Growth Inhibition Assay | IC50=7.76159 μM | SANGER | |||
| OVCAR-4 | Growth Inhibition Assay | IC50=9.11675 μM | SANGER | |||
| D-336MG | Growth Inhibition Assay | IC50=9.47395 μM | SANGER | |||
| GCIY | Growth Inhibition Assay | IC50=9.5742 μM | SANGER | |||
| KS-1 | Growth Inhibition Assay | IC50=9.66287 μM | SANGER | |||
| HCC2998 | Growth Inhibition Assay | IC50=9.96307 μM | SANGER | |||
| D-247MG | Growth Inhibition Assay | IC50=9.98291 μM | SANGER | |||
| TE-15 | Growth Inhibition Assay | IC50=10.245 μM | SANGER | |||
| IST-MES1 | Growth Inhibition Assay | IC50=10.2565 μM | SANGER | |||
| ETK-1 | Growth Inhibition Assay | IC50=10.623 μM | SANGER | |||
| RCC10RGB | Growth Inhibition Assay | IC50=10.961 μM | SANGER | |||
| KNS-42 | Growth Inhibition Assay | IC50=11.7255 μM | SANGER | |||
| LB771-HNC | Growth Inhibition Assay | IC50=12.1712 μM | SANGER | |||
| SR | Growth Inhibition Assay | IC50=12.2064 μM | SANGER | |||
| NCI-H1355 | Growth Inhibition Assay | IC50=12.8985 μM | SANGER | |||
| ES6 | Growth Inhibition Assay | IC50=13.078 μM | SANGER | |||
| SK-NEP-1 | Growth Inhibition Assay | IC50=13.2577 μM | SANGER | |||
| D-392MG | Growth Inhibition Assay | IC50=13.6428 μM | SANGER | |||
| NB7 | Growth Inhibition Assay | IC50=14.2374 μM | SANGER | |||
| SK-LMS-1 | Growth Inhibition Assay | IC50=14.5145 μM | SANGER | |||
| SK-UT-1 | Growth Inhibition Assay | IC50=14.7889 μM | SANGER | |||
| CA46 | Growth Inhibition Assay | IC50=15.0586 μM | SANGER | |||
| IST-SL2 | Growth Inhibition Assay | IC50=15.1901 μM | SANGER | |||
| BC-1 | Growth Inhibition Assay | IC50=15.3314 μM | SANGER | |||
| LS-123 | Growth Inhibition Assay | IC50=15.8173 μM | SANGER | |||
| Ramos-2G6-4C10 | Growth Inhibition Assay | IC50=16.0924 μM | SANGER | |||
| MZ1-PC | Growth Inhibition Assay | IC50=16.7313 μM | SANGER | |||
| LB647-SCLC | Growth Inhibition Assay | IC50=16.9372 μM | SANGER | |||
| NCI-H1694 | Growth Inhibition Assay | IC50=17.1529 μM | SANGER | |||
| NCI-H322M | Growth Inhibition Assay | IC50=17.4366 μM | SANGER | |||
| ES7 | Growth Inhibition Assay | IC50=18.3914 μM | SANGER | |||
| LC-2-ad | Growth Inhibition Assay | IC50=18.4386 μM | SANGER | |||
| SF268 | Growth Inhibition Assay | IC50=18.7409 μM | SANGER | |||
| RPMI-8402 | Growth Inhibition Assay | IC50=19.0742 μM | SANGER | |||
| HCE-T | Growth Inhibition Assay | IC50=20.2344 μM | SANGER | |||
| A101D | Growth Inhibition Assay | IC50=20.8587 μM | SANGER | |||
| MRK-nu-1 | Growth Inhibition Assay | IC50=20.913 μM | SANGER | |||
| LXF-289 | Growth Inhibition Assay | IC50=21.038 μM | SANGER | |||
| NALM-6 | Growth Inhibition Assay | IC50=21.1967 μM | SANGER | |||
| DOHH-2 | Growth Inhibition Assay | IC50=21.4813 μM | SANGER | |||
| EW-16 | Growth Inhibition Assay | IC50=22.1402 μM | SANGER | |||
| A4-Fuk | Growth Inhibition Assay | IC50=22.2149 μM | SANGER | |||
| HD-MY-Z | Growth Inhibition Assay | IC50=22.3965 μM | SANGER | |||
| SKM-1 | Growth Inhibition Assay | IC50=22.7351 μM | SANGER | |||
| DMS-153 | Growth Inhibition Assay | IC50=23.4204 μM | SANGER | |||
| LB373-MEL-D | Growth Inhibition Assay | IC50=23.5452 μM | SANGER | |||
| LP-1 | Growth Inhibition Assay | IC50=23.8097 μM | SANGER | |||
| GI-ME-N | Growth Inhibition Assay | IC50=24.292 μM | SANGER | |||
| MPP-89 | Growth Inhibition Assay | IC50=25.2036 μM | SANGER | |||
| U-698-M | Growth Inhibition Assay | IC50=25.2503 μM | SANGER | |||
| HC-1 | Growth Inhibition Assay | IC50=25.6418 μM | SANGER | |||
| HCC2157 | Growth Inhibition Assay | IC50=25.673 μM | SANGER | |||
| MOLT-4 | Growth Inhibition Assay | IC50=26.273 μM | SANGER | |||
| LS-411N | Growth Inhibition Assay | IC50=26.3369 μM | SANGER | |||
| Becker | Growth Inhibition Assay | IC50=26.5181 μM | SANGER | |||
| NCI-H23 | Growth Inhibition Assay | IC50=26.7575 μM | SANGER | |||
| IST-SL1 | Growth Inhibition Assay | IC50=27.3867 μM | SANGER | |||
| MZ2-MEL | Growth Inhibition Assay | IC50=27.4566 μM | SANGER | |||
| RKO | Growth Inhibition Assay | IC50=28.1446 μM | SANGER | |||
| TE-441-T | Growth Inhibition Assay | IC50=28.789 μM | SANGER | |||
| EW-24 | Growth Inhibition Assay | IC50=29.1259 μM | SANGER | |||
| no-10 | Growth Inhibition Assay | IC50=29.1631 μM | SANGER | |||
| D-542MG | Growth Inhibition Assay | IC50=29.9221 μM | SANGER | |||
| ST486 | Growth Inhibition Assay | IC50=30.6451 μM | SANGER | |||
| KURAMOCHI | Growth Inhibition Assay | IC50=30.8057 μM | SANGER | |||
| ES8 | Growth Inhibition Assay | IC50=31.5972 μM | SANGER | |||
| BL-41 | Growth Inhibition Assay | IC50=32.1054 μM | SANGER | |||
| NB6 | Growth Inhibition Assay | IC50=32.3855 μM | SANGER | |||
| NCI-H1304 | Growth Inhibition Assay | IC50=32.4967 μM | SANGER | |||
| MS-1 | Growth Inhibition Assay | IC50=32.7751 μM | SANGER | |||
| MFH-ino | Growth Inhibition Assay | IC50=34.3224 μM | SANGER | |||
| NOS-1 | Growth Inhibition Assay | IC50=34.6748 μM | SANGER | |||
| HUTU-80 | Growth Inhibition Assay | IC50=35.3667 μM | SANGER | |||
| EB2 | Growth Inhibition Assay | IC50=36.6189 μM | SANGER | |||
| L-540 | Growth Inhibition Assay | IC50=37.2308 μM | SANGER | |||
| NCI-H747 | Growth Inhibition Assay | IC50=38.8846 μM | SANGER | |||
| NCI-H446 | Growth Inhibition Assay | IC50=39.9651 μM | SANGER | |||
| MOLT-16 | Growth Inhibition Assay | IC50=42.415 μM | SANGER | |||
| BC-3 | Growth Inhibition Assay | IC50=45.4896 μM | SANGER | |||
| SJSA-1 | Growth Inhibition Assay | IC50=45.5474 μM | SANGER | |||
| BB65-RCC | Growth Inhibition Assay | IC50=45.666 μM | SANGER | |||
| SNB75 | Growth Inhibition Assay | IC50=46.018 μM | SANGER | |||
| SKBR3 | Cytotoxicity assay | Cytotoxicity against human SKBR3 cells overexpressing HER2 by resazurin dye reduction assay, IC50 = 0.002 μM. | 21080629 | |||
| SKOV3 | Cytotoxicity assay | Cytotoxicity against human SKOV3 cells overexpressing HER2 by resazurin dye reduction assay, IC50 = 0.003 μM. | 21080629 | |||
| CAL27 | Cytotoxicity assay | Cytotoxicity against human CAL27 cells overexpressing EGFR by resazurin dye reduction assay, IC50 = 0.007 μM. | 21080629 | |||
| 4T1 | Cytotoxicity assay | Cytotoxicity against mouse triple negative 4T1 cells, IC50 = 0.01037 μM. | 24890652 | |||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MCF7 cells after 48 hrs by SRB assay, IC50 = 0.011 μM. | 25151582 | ||
| SKBR3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human SKBR3 cells after 72 hrs by SRB assay, IC50 = 0.017 μM. | 19028425 | ||
| MDA-MB-231 | Cytotoxicity assay | Cytotoxicity against human triple negative MDA-MB-231 cells, IC50 = 0.02 μM. | 24890652 | |||
| BT474 | Antiproliferative assay | Antiproliferative activity against human BT474 cells overexpressing ErB2, IC50 = 0.025 μM. | 18653333 | |||
| BT474 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human BT474 cells after 72 hrs by methylene blue staining-based method, IC50 = 0.025 μM. | 21887403 | ||
| HEK293 | Function assay | Inhibition of recombinant human C-terminal His-tagged EGFR (1 to 645 residues) expressed in HEK293 cells by ELISA, IC50 = 0.02706 μM. | 28711703 | |||
| HEK293 | Function assay | Inhibition of recombinant human N-terminal His-tagged EGFR (1 to 645 residues) expressed in HEK293 cells by ELISA, IC50 = 0.0271 μM. | 29421573 | |||
| HepG2 | Function assay | Inhibition of EGFR in human HepG2 cells, IC50 = 0.0271 μM. | 30096580 | |||
| SKBR3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human SKBR3 cells after 72 hrs by SRB assay, IC50 = 0.029 μM. | 19888761 | ||
| SKBR3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human SKBR3 cells overexpressing HER-2 gene after 72 hrs by SRB assay, IC50 = 0.029 μM. | 22372864 | ||
| MDA-MB-435 | Cytotoxicity assay | Cytotoxicity against human MDA-MB-435 cells, IC50 = 0.0297 μM. | 24890652 | |||
| SKBR3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human SKBR3 cells assessed as inhibition of cell growth after 72 hrs by sulforhodamine B assay, IC50 = 0.0301 μM. | 25305330 | ||
| BT474 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human BT474 cells expressing Her2 assessed as reduction in cell viability after 72 hrs by cell titer-glo luminescence assay, IC50 = 0.031 μM. | 27288180 | ||
| CAL27 | Function assay | 16 hrs | Inhibition of EGF-induced EGFR phosphorylation in human CAL27 cells overexpressing EGFR after 16 hrs by Western blot, IC50 = 0.032 μM. | 21080629 | ||
| NCI-N87 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human NCI-N87 cells expressing HER-2 after 72 hrs by SRB assay, IC50 = 0.036 μM. | 22372864 | ||
| insect cells | Function assay | 5 mins | Inhibition of recombinant human EGFR L858R mutant expressed in baculovirus infected insect cells preincubated for 5 mins followed by ATP addition and measured after 30 mins by HTRF assay, IC50 = 0.03755 μM. | 30096580 | ||
| SK-BR-3 | Antiproliferative assay | Antiproliferative activity against human SK-BR-3 cells after hrs by ATP content assay, IC50 = 0.04 μM. | 20143778 | |||
| MDA-MB-175 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MDA-MB-175 cells expressing Src, Ret and low HER-2 after 72 hrs by SRB assay, IC50 = 0.0444 μM. | 22372864 | ||
| A431 | Function assay | Inhibition of EGFR intracellular phosphorylation in human A431 cells by ELISA, IC50 = 0.052 μM. | 20346655 | |||
| SK-BR-3 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human SK-BR-3 cells expressing HER2 assessed as reduction in cell viability measured after 48 hrs by MTT assay, IC50 = 0.06 μM. | 27769671 | ||
| BT474 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human BT474 cells assessed as inhibition of cell growth after 72 hrs by sulforhodamine B assay, IC50 = 0.0639 μM. | 25305330 | ||
| MCF7 | Cytotoxicity assay | Cytotoxicity against human ER-positive MCF7 cells, IC50 = 0.0649 μM. | 24890652 | |||
| MDA-MB-468 | Cytotoxicity assay | Cytotoxicity against human triple negative MDA-MB-468 cells, IC50 = 0.0832 μM. | 24890652 | |||
| NCI-N87 | Antiproliferative assay | 3 days | Antiproliferative activity against human NCI-N87 cells after 3 days by methylene blue staining, IC50 = 0.09 μM. | 22101132 | ||
| N87 | Function assay | Inhibition of ErbB2 intracellular phosphorylation in human N87 cells by ELISA, IC50 = 0.1 μM. | 20346655 | |||
| BT474 | Antiproliferative assay | 3 days | Antiproliferative activity against human BT474 cells after 3 days by methylene blue staining, IC50 = 0.1 μM. | 22101132 | ||
| Calu3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human Calu3 cells after 72 hrs by MTT assay, IC50 = 0.1 μM. | 28092860 | ||
| BT474 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human BT474 cells expressing HER2 assessed as reduction in cell viability measured after 48 hrs by MTT assay, IC50 = 0.1 μM. | 27769671 | ||
| A431 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A431 cells after 72 hrs by SRB assay, IC50 = 0.104 μM. | 19888761 | ||
| A431 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A431 cells overexpressing HER-1 gene after 72 hrs by SRB assay, IC50 = 0.104 μM. | 22372864 | ||
| HeLa | Antiproliferative assay | 12 hrs | Antiproliferative activity against human HeLa cells after 12 hrs by MTT assay, IC50 = 0.12 μM. | 26652482 | ||
| MIAPaCa | Function assay | Inhibition of ERBb2 phosphorylation in human MIAPaCa cells by ELISA, IC50 = 0.14 μM. | 20817523 | |||
| A431 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human A431 cells expressing EGFR/HER2 assessed as reduction in cell viability measured after 48 hrs by MTT assay, IC50 = 0.15 μM. | 27769671 | ||
| MDA-MB-453 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MDA-MB-453 cells assessed as inhibition of cell growth after 72 hrs by sulforhodamine B assay, IC50 = 0.19 μM. | 25305330 | ||
| BT474 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human BT474 cells assessed as growth inhibition after 72 hrs by calcein-AM dye based fluorescence assay, GI50 = 0.2 μM. | 29089259 | ||
| B16F10 | Antiproliferative assay | 12 hrs | Antiproliferative activity against mouse B16F10 cells after 12 hrs by MTT assay, IC50 = 0.21 μM. | 26652482 | ||
| Sf21 | Function assay | 5 mins | Inhibition of N-terminal GST-tagged recombinant human EGFR T790M mutant expressed in baculovirus infected Sf21 insect cells preincubated for 5 mins followed by ATP addition and measured after 30 mins by HTRF assay, IC50 = 0.22489 μM. | 30096580 | ||
| Calu3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human Calu3 cells assessed as inhibition of cell growth after 72 hrs by sulforhodamine B assay, IC50 = 0.23 μM. | 25305330 | ||
| DIFI | Cytotoxicity assay | 4 days | Cytotoxicity against human DIFI cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 0.235 μM. | 22169601 | ||
| HepG2 | Antiproliferative assay | 12 hrs | Antiproliferative activity against human HepG2 cells after 12 hrs by MTT assay, IC50 = 0.27 μM. | 26652482 | ||
| EOL-1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human EOL-1 cells assessed as growth inhibition after 72 hrs by calcein-AM dye based fluorescence assay, GI50 = 0.41 μM. | 29089259 | ||
| MIAPaCa | Function assay | Inhibition of EGFR phosphorylation in human MIAPaCa cells by ELISA, IC50 = 0.433 μM. | 20817523 | |||
| MCF7 | Antiproliferative assay | 12 hrs | Antiproliferative activity against human MCF7 cells after 12 hrs by MTT assay, IC50 = 0.47 μM. | 26652482 | ||
| SKBR3 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human SKBR3 cells after 48 hrs by MTT assay, IC50 = 0.49 μM. | 27187856 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells expressing EGFR and KRAS mutant assessed as reduction in cell viability measured after 48 hrs by MTT assay, IC50 = 0.51 μM. | 27769671 | ||
| CAL27 | Cytotoxicity assay | 4 days | Cytotoxicity against human CAL27 cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 0.53 μM. | 22169601 | ||
| MDA-MB-453 | Cytotoxicity assay | 72 hrs | Cytotoxicity against PTEN-deficient human MDA-MB-453 cells expressing HER-2, and PIK3CA mutant after 72 hrs by SRB assay, IC50 = 0.555 μM. | 22372864 | ||
| SKOV3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human SKOV3 cells assessed as inhibition of cell growth after 72 hrs by sulforhodamine B assay, IC50 = 0.59 μM. | 25305330 | ||
| HCC827 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCC827 cells harboring EGFR E746-A750 del mutant after 72 hrs by MTT assay, IC50 = 0.6 μM. | 29421573 | ||
| A431NS | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A431NS cells after 72 hrs by MTT assay, IC50 = 0.6 μM. | 28092860 | ||
| A431 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A431 cells assessed as inhibition of cell growth after 72 hrs by sulforhodamine B assay, IC50 = 0.6431 μM. | 25305330 | ||
| BT474 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human BT474 cells expressing HER2 after 72 hrs by Cell Titer Glo assay, GI50 = 0.93 μM. | 26475520 | ||
| A431 | Cytotoxicity assay | Cytotoxicity against human A431 cells overexpressing EGFR by resazurin dye reduction assay, IC50 = 0.97 μM. | 21080629 | |||
| MDA-MB-361 | Cytotoxicity assay | 72 hrs | Cytotoxicity against ER activated human MDA-MB-361 cells expressing Her-2, Src and PIK3CA mutant after 72 hrs by SRB assay, IC50 = 1.029 μM. | 22372864 | ||
| GXF251L | Cytotoxicity assay | 4 days | Cytotoxicity against human GXF251L cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 1.48 μM. | 22169601 | ||
| A431 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A431 cells overexpressing EGFR after 72 hrs by MTT assay, IC50 = 2.6 μM. | 22595177 | ||
| A431 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A431 cells overexpressing EGFR after 72 hrs by MTT assay, IC50 = 2.62 μM. | 22182581 | ||
| LXFA 629L | Cytotoxicity assay | 4 days | Cytotoxicity against human LXFA 629L cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 2.87 μM. | 22169601 | ||
| DU145 | Cytotoxicity assay | 4 days | Cytotoxicity against human DU145 cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 2.99 μM. | 22169601 | ||
| SKOV3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human SKOV3 cells overexpressing ErbB2 after 72 hrs by MTT assay, IC50 = 2.99 μM. | 22182581 | ||
| NCI-H1781 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human NCI-H1781 cells expressing HER-2 G776insV_G/C after 72 hrs by SRB assay, IC50 = 3.047 μM. | 22372864 | ||
| OVXF 899L | Cytotoxicity assay | 4 days | Cytotoxicity against human OVXF 899L cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 3.35 μM. | 22169601 | ||
| LXFL 529L | Cytotoxicity assay | 4 days | Cytotoxicity against human LXFL 529L cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 3.51 μM. | 22169601 | ||
| LNCAP | Cytotoxicity assay | 4 days | Cytotoxicity against human LNCAP cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 3.68 μM. | 22169601 | ||
| Bel7402 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human Bel7402 cells after 72 hrs by MTT assay, IC50 = 3.7 μM. | 28092860 | ||
| LXFA 526L | Cytotoxicity assay | 4 days | Cytotoxicity against human LXFA 526L cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 4.21 μM. | 22169601 | ||
| NIH/3T3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against mouse NIH/3T3 cells after 72 hrs by MTT assay, IC50 = 4.3 μM. | 22595177 | ||
| SK-BR-3 | Antiproliferative assay | Antiproliferative activity against ErbB2 overexpressing human SK-BR-3 cells by MTT assay, IC50 = 4.35 μM. | 21570843 | |||
| UXF 1138L | Cytotoxicity assay | 4 days | Cytotoxicity against human UXF 1138L cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 4.4 μM. | 22169601 | ||
| SK-BR-3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SK-BR-3 cells after 72 hrs by MTT assay, IC50 = 4.4 μM. | 28092860 | ||
| PC3M | Cytotoxicity assay | 4 days | Cytotoxicity against human PC3M cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 4.55 μM. | 22169601 | ||
| COLO205 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human COLO205 cells after 72 hrs by MTT assay, IC50 = 4.6 μM. | 28092860 | ||
| HT-29 | Cytotoxicity assay | 4 days | Cytotoxicity against human HT-29 cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 4.62 μM. | 22169601 | ||
| A431 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A431 cells harboring wild type EGFR after 72 hrs by MTT assay, IC50 = 4.8 μM. | 29421573 | ||
| A431 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A431 cells harboring wild type EGFR after 72 hrs by MTT assay, IC50 = 4.8 μM. | 28711703 | ||
| A431 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A431 cells assessed as decrease in cell viability after 72 hrs by MTT assay, IG50 = 4.8 μM. | 28238614 | ||
| MCF7 | Cytotoxicity assay | 4 days | Cytotoxicity against human MCF7 cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 4.83 μM. | 22169601 | ||
| MEXF 1341L | Cytotoxicity assay | 4 days | Cytotoxicity against human MEXF 1341L cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 4.85 μM. | 22169601 | ||
| MCF7 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human MCF7 cells after 24 hrs by SRB assay, IC50 = 5.02 μM. | 30096580 | ||
| SKBR3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human estrogen receptor and progesterone receptor deficient SKBR3 cells expressing HER2 after 72 hrs by MTT assay, IC50 = 5.05 μM. | 23927972 | ||
| SKHEP1 | Antiproliferative assay | hrs | Antiproliferative activity against human SKHEP1 cells after hrs by ATP content assay, IC50 = 5.3 μM. | 20143778 | ||
| RKO | Cytotoxicity assay | 4 days | Cytotoxicity against human RKO cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 5.35 μM. | 22169601 | ||
| MDA-MB-231 | Antiproliferative assay | hrs | Antiproliferative activity against human MDA-MB-231 cells after hrs by ATP content assay, IC50 = 5.4 μM. | 20143778 | ||
| Hep3B2 | Antiproliferative assay | hrs | Antiproliferative activity against human Hep3B2 cells after hrs by ATP content assay, IC50 = 5.49 μM. | 20143778 | ||
| K562 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human K562 cells assessed as growth inhibition after 72 hrs by calcein-AM dye based fluorescence assay, GI50 = 5.7 μM. | 29089259 | ||
| LXFA 289L | Cytotoxicity assay | 4 days | Cytotoxicity against human LXFA 289L cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 5.79 μM. | 22169601 | ||
| MAXF 401NL | Cytotoxicity assay | 4 days | Cytotoxicity against human MAXF 401NL cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 5.8 μM. | 22169601 | ||
| HeLa | Cytotoxicity assay | Cytotoxicity against human HeLa cells overexpressing HDAC by resazurin dye reduction assay, IC50 = 5.9 μM. | 21080629 | |||
| HCT116 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human HCT116 cells after 24 hrs by SRB assay, IC50 = 5.92 μM. | 30096580 | ||
| 22Rv1 | Cytotoxicity assay | 4 days | Cytotoxicity against human 22Rv1 cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 6.06 μM. | 22169601 | ||
| PAXF 546L | Cytotoxicity assay | 4 days | Cytotoxicity against human PAXF 546L cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 6.12 μM. | 22169601 | ||
| HCT116 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT116 cells after 72 hrs by MTT assay, IC50 = 6.2 μM. | 28092860 | ||
| HepG2 | Antiproliferative assay | Antiproliferative activity against human HepG2 cells after hrs by ATP content assay, IC50 = 6.27 μM. | 20143778 | |||
| HepG2 | Cytotoxicity assay | Cytotoxicity against human HepG2 cells assessed as reduction in cell viability, TC50 = 6.27 μM. | 29049963 | |||
| HepG2 | Cytotoxicity assay | Cytotoxicity against human HepG2 cells, TC50 = 6.3 μM. | 30344906 | |||
| NCI-H522 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human NCI-H522 cells after 72 hrs by MTT assay, IC50 = 6.4 μM. | 28092860 | ||
| MCF7 | Antiproliferative assay | hrs | Antiproliferative activity against human MCF7 cells after hrs by ATP content assay, IC50 = 6.6 μM. | 20143778 | ||
| A549 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human A549 cells after 48 hrs by MTT assay, IC50 = 6.74 μM. | 27187856 | ||
| MCF7 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MCF7 cells after 72 hrs by MTT assay, IC50 = 6.8 μM. | 29684708 | ||
| PXF 1118L | Cytotoxicity assay | 4 days | Cytotoxicity against human PXF 1118L cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 6.86 μM. | 22169601 | ||
| LIXF 575L | Cytotoxicity assay | 4 days | Cytotoxicity against human LIXF 575L cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 7.18 μM. | 22169601 | ||
| RXF 1781L | Cytotoxicity assay | 4 days | Cytotoxicity against human RXF 1781L cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 7.67 μM. | 22169601 | ||
| MDA231 | Cytotoxicity assay | 4 days | Cytotoxicity against human MDA231 cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 7.7 μM. | 22169601 | ||
| LXFL 1121L | Cytotoxicity assay | 4 days | Cytotoxicity against human LXFL 1121L cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 7.73 μM. | 22169601 | ||
| RXF 393NL | Cytotoxicity assay | 4 days | Cytotoxicity against human RXF 393NL cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 7.77 μM. | 22169601 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells assessed as growth inhibition after 72 hrs by calcein-AM dye based fluorescence assay, GI50 = 8.1 μM. | 29089259 | ||
| PANC1 | Cytotoxicity assay | 4 days | Cytotoxicity against human PANC1 cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 8.12 μM. | 22169601 | ||
| CXF 269L | Cytotoxicity assay | 4 days | Cytotoxicity against human CXF 269L cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 8.36 μM. | 22169601 | ||
| NCI-H460 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human NCI-H460 cells after 72 hrs by MTT assay, IC50 = 8.4 μM. | 28092860 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells assessed as inhibition of cell growth after 72 hrs by sulforhodamine B assay, IC50 = 8.468 μM. | 25305330 | ||
| A549 | Cytotoxicity assay | Cytotoxicity against human A549 cells overexpressing HDAC by resazurin dye reduction assay, IC50 = 8.5 μM. | 21080629 | |||
| NCI-H1975 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human NCI-H1975 cells harboring EGFR L858R/T790M mutant after 72 hrs by MTT assay, IC50 = 9.08 μM. | 28711703 | ||
| NCI-H1975 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human NCI-H1975 cells assessed as decrease in cell viability after 72 hrs by MTT assay, IG50 = 9.08 μM. | 28238614 | ||
| RXF 486L | Cytotoxicity assay | 4 days | Cytotoxicity against human RXF 486L cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 9.1 μM. | 22169601 | ||
| Saos2 | Cytotoxicity assay | 4 days | Cytotoxicity against human Saos2 cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 9.38 μM. | 22169601 | ||
| PXF 1752L | Cytotoxicity assay | 4 days | Cytotoxicity against human PXF 1752L cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 9.46 μM. | 22169601 | ||
| PXF 698L | Cytotoxicity assay | 4 days | Cytotoxicity against human PXF 698L cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 9.49 μM. | 22169601 | ||
| BXF T24 | Cytotoxicity assay | 4 days | Cytotoxicity against human BXF T24 cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 9.65 μM. | 22169601 | ||
| HCT116 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT116 cells after 72 hrs by MTT assay, IC50 = 10.12 μM. | 29684708 | ||
| MEXF 462NL | Cytotoxicity assay | 4 days | Cytotoxicity against human MEXF 462NL cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 10.13 μM. | 22169601 | ||
| A2780 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A2780 cells after 72 hrs by MTT assay, IC50 = 10.4 μM. | 29684708 | ||
| TE671 | Cytotoxicity assay | 4 days | Cytotoxicity against human TE671 cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 10.95 μM. | 22169601 | ||
| RAW264.7 | Antileishmanial assay | 96 hrs | Antileishmanial activity against luciferase-expressing Leishmania major amastigotes infected in mouse RAW264.7 cells after 96 hrs by luminescence assay, EC50 = 11 μM. | 28337329 | ||
| PAXF 1657L | Cytotoxicity assay | 4 days | Cytotoxicity against human PAXF 1657L cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 11.11 μM. | 22169601 | ||
| MEXF 276L | Cytotoxicity assay | 4 days | Cytotoxicity against human MEXF 276L cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 11.37 μM. | 22169601 | ||
| MKN45 | Cytotoxicity assay | 4 days | Cytotoxicity against human MKN45 cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 11.48 μM. | 22169601 | ||
| BXF 1352L | Cytotoxicity assay | 4 days | Cytotoxicity against human BXF 1352L cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 11.53 μM. | 22169601 | ||
| HepG2 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human HepG2 cells after 24 hrs by SRB assay, IC50 = 11.71 μM. | 30096580 | ||
| BXF 1218L | Cytotoxicity assay | 4 days | Cytotoxicity against human BXF 1218L cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 11.77 μM. | 22169601 | ||
| Caco | Cytotoxicity assay | 24 hrs | Cytotoxicity against human Caco cells after 24 hrs by SRB assay, IC50 = 12.11 μM. | 30096580 | ||
| SW480 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SW480 cells harboring wild type EGFR after 72 hrs by MTT assay, IC50 = 12.58 μM. | 29421573 | ||
| SW480 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SW480 cells harboring wild type EGFR after 72 hrs by MTT assay, IC50 = 12.58 μM. | 28711703 | ||
| SW480 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human SW480 cells assessed as decrease in cell viability after 72 hrs by MTT assay, IG50 = 12.58 μM. | 28238614 | ||
| NCI-H1975 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human NCI-H1975 cells harboring EGFR L858R/T790M double mutant after 72 hrs by MTT assay, IC50 = 12.68 μM. | 29421573 | ||
| 3T3 | Antitrypanosomal assay | 48 hrs | Antitrypanosomal activity against Trypanosoma cruzi Tulahuen amastigotes infected in rat 3T3 cells after 48 hrs by chlorophenol red-beta-D-galactopyranoside based assay, EC50 = 13 μM. | 28337329 | ||
| HCT116 | Cytotoxicity assay | 4 days | Cytotoxicity against human HCT116 cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 13.14 μM. | 22169601 | ||
| OVCAR3 | Cytotoxicity assay | 4 days | Cytotoxicity against human OVCAR3 cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 13.44 μM. | 22169601 | ||
| H460 | Cytotoxicity assay | 4 days | Cytotoxicity against human H460 cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50 = 13.46 μM. | 22169601 | ||
| A549 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A549 cells harboring wild type EGFR/K-ras mutant after 72 hrs by MTT assay, IC50 = 14.9 μM. | 29421573 | ||
| A549 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A549 cells harboring wild type EGFR/K-Ras mutant after 72 hrs by MTT assay, IC50 = 14.9 μM. | 28711703 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells assessed as decrease in cell viability after 72 hrs by MTT assay, IG50 = 14.9 μM. | 28238614 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MCF7 cells expressing pcDNA3 assessed as reduction of cell viability after 48 hrs CellTiter-Glo assay, IC50 = 15.71 μM. | 24355130 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MCF7 cells expressing HER2 assessed as reduction of cell viability after 48 hrs CellTiter-Glo assay, IC50 = 15.79 μM. | 24355130 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MCF7 cells expressing HER2delta16 assessed as reduction of cell viability after 48 hrs CellTiter-Glo assay, IC50 = 19.22 μM. | 24355130 | ||
| MDA-MB-468 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MDA-MB-468 cells after 72 hrs by MTT assay, IC50 = 20 μM. | 28092860 | ||
| HCC827 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCC827 cells assessed as growth inhibition after 72 hrs by calcein-AM dye based fluorescence assay, GI50 = 22.3 μM. | 29089259 | ||
| MDA-MB-468 | Antiproliferative assay | Antiproliferative activity against EGFR overexpressing human MDA-MB-468 cells by MTT assay, IC50 = 23.46 μM. | 21570843 | |||
| HCT116 | Antiproliferative assay | Antiproliferative activity against human HCT116 cells by MTT assay, IC50 = 42.36 μM. | 21570843 | |||
| NCI-N87 | Antitumor assay | Antitumor activity against human NCI-N87 cells xenografted in athymic mouse assessed as tumor growth inhibition at 20 mg/kg, po QD for 14 days measured after 19 days | 22101132 | |||
| A431 | Function assay | 0.001 to 10 uM | 2 hrs | Inhibition of HER1 (unknown origin) expressed in human A431 cells assessed as reduction in ERK1/2 phosphorylation at 0.001 to 10 uM incubated for 2 hrs by Western blot method | 25305330 | |
| A431 | Function assay | 0.001 to 10 uM | 2 hrs | Inhibition of HER1 (unknown origin) expressed in human A431 cells assessed as reduction in AKT phosphorylation at 0.001 to 10 uM incubated for 2 hrs by Western blot method | 25305330 | |
| BT474 | Function assay | 0.001 to 10 uM | 2 hrs | Inhibition of HER2 (unknown origin) expressed in human BT474 cells assessed as reduction in ERK1/2 phosphorylation at 0.001 to 10 uM incubated for 2 hrs by Western blot method | 25305330 | |
| BT474 | Function assay | 0.001 to 10 uM | 2 hrs | Inhibition of HER2 (unknown origin) expressed in human BT474 cells assessed as reduction in AKT phosphorylation at 0.001 to 10 uM incubated for 2 hrs by Western blot method | 25305330 | |
| A431 | Function assay | 0.001 to 10 uM | 2 hrs | Inhibition of HER1 phosphorylation (unknown origin) expressed in human A431 cells at 0.001 to 10 uM incubated for 2 hrs by Western blot method | 25305330 | |
| BT474 | Function assay | 0.001 to 10 uM | 2 hrs | Inhibition of HER2 phosphorylation (unknown origin) expressed in human BT474 cells at 0.001 to 10 uM incubated for 2 hrs by Western blot method | 25305330 | |
| U-2 OS | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for U-2 OS cells | 29435139 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | |||
| DAOY | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for DAOY cells | 29435139 | |||
| Saos-2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Saos-2 cells | 29435139 | |||
| BT-37 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-37 cells | 29435139 | |||
| BT-37 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-37 cells | 29435139 | |||
| RD | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for RD cells | 29435139 | |||
| RD | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for RD cells | 29435139 | |||
| SK-N-SH | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-SH cells | 29435139 | |||
| MG 63 (6-TG R) | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for MG 63 (6-TG R) cells | 29435139 | |||
| BT-12 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for BT-12 cells | 29435139 | |||
| SK-N-SH | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for SK-N-SH cells | 29435139 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for A673 cells) | 29435139 | |||
| Rh30 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for Rh30 cells | 29435139 | |||
| U-2 OS | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for U-2 OS cells | 29435139 | |||
| Rh41 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for Rh41 cells | 29435139 | |||
| SJ-GBM2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SJ-GBM2 cells | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 29435139 | |||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 29435139 | |||
| LAN-5 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for LAN-5 cells | 29435139 | |||
| Rh18 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh18 cells | 29435139 | |||
| Rh18 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for Rh18 cells | 29435139 | |||
| NCI-H522 | Antitumor assay | Antitumor activity against human NCI-H522 cells xenografted in Balb/c nude mouse assessed as tumor growth inhibition at 100 mg/kg, ig administered once daily for 28 days relative to control | 28092860 | |||
| MCF7 | Function assay | 10 uM | 2 hrs | Inhibition of HER2 delta16 mutant autophosphorylation at Y1248 in human MCF7 cells at 10 uM after 2 hrs by Western blotting | 24355130 | |
| MCF7 | Function assay | 10 uM | 2 hrs | Inhibition of HER2 autophosphorylation at Y1248 in human MCF7 cells expressing pcDNA3 at 10 uM after 2 hrs by Western blotting | 24355130 | |
| MCF7 | Function assay | 10 uM | 2 hrs | Inhibition of EGFR autophosphorylation at Y1068 in human MCF7 cells expressing HER2delta16 at 10 uM after 2 hrs by Western blotting | 24355130 | |
| MCF7 | Function assay | 10 uM | 2 hrs | Inhibition of EGFR autophosphorylation at Y1068 in human MCF7 cells expressing HER2 at 10 uM after 2 hrs by Western blotting | 24355130 | |
| MCF7 | Function assay | 10 uM | 2 hrs | Inhibition of EGFR autophosphorylation at Y1068 in human MCF7 cells expressing pcDNA3 at 10 uM after 2 hrs by Western blotting | 24355130 | |
| MCF7 | Function assay | 10 uM | 2 hrs | Inhibition of HER2 autophosphorylation at Y1248 in human MCF7 cells at 10 uM after 2 hrs by Western blotting | 24355130 | |
| A549 | Function assay | 50 uM | 2 hrs | Inhibition of EGFR phosphorylation at Y1068 in human A549 cells at 50 uM preincubated for 2 hrs followed by EGF induction measured after 10 mins by Western blot method | 28238614 | |
| A549 | Function assay | 50 uM | 2 hrs | Inhibition of EGFR phosphorylation at Tyr residue in human A549 cells at 50 uM preincubated for 2 hrs followed by EGF induction measured after 10 mins by Western blot method | 28238614 | |
| LLC | Antitumor assay | Antitumor activity against mouse LLC cells implanted in in C57BL/6 mouse assessed as tumor growth inhibition at 100 mg/kg, po qd for 14 days | 27187856 | |||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(172.09 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 581.06 | Formula | C29H26ClFN4O4S |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 231277-92-2 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | GSK572016, GW2016 | Smiles | CS(=O)(=O)CCNCC1=CC=C(O1)C2=CC3=C(C=C2)N=CN=C3NC4=CC(=C(C=C4)OCC5=CC(=CC=C5)F)Cl | ||
Mechanism of Action
| Targets/IC50/Ki |
ErbB2
(Cell-free assay) 9.2 nM
EGFR
(Cell-free assay) 10.8 nM
ErbB4
(Cell-free assay) 367 nM
|
|---|---|
| In vitro |
Lapatinib weakly inhibits the activity of ErbB4 with IC50 of 367 nM, and displays >300-fold selectivity for EGFR and ErbB2 over other kinases such as c-Src, c-Raf, MEK, ERK, c-Fms, CDK1, CDK2, p38, Tie-2, and VEGFR2. This compound significantly inhibits receptor autophosphorylation of EGFR and ErbB2 in a dose-dependent manner with IC50 of 170 nM and 80 nM, respectively in HN5 cells; as well as 210 nM and 60 nM, respectively in BT474 cells. Unlike OSI-774 and Iressa (ZD1839) which preferentially inhibit the growth of the EGFR-overexpressing cells, it inhibits the growth of both EGFR- and ErbB2-overexpressing cells. This chemical displays higher inhibitory activity against EGFR- or ErbB2-overexpressing cells with IC50 of 0.09-0.21 μM, compared with cells expressing low levels of EGFR or ErbB2 with IC50 of 3-12 μM, and exhibits ~100-fold selectivity over the normal fibroblast cells. It potently inhibits the outgrowth of EGFR-overexpressing HN5 and A-431 cells, as well as ErbB2-overexpressing BT474 and N87 cells, and significantly induces G1 arrest of HN5 cells and apoptosis of BT474 cells, which are associated with inhibition of AKT phosphorylation.
|
| Kinase Assay |
In vitro kinase assays
|
|
The IC50 values for inhibition of enzyme activity are generated by measuring inhibition of phosphorylation of a peptide substrate. The intracellular kinase domains of EGFR and ErbB2 are purified from a baculovirus expression system. EGFR and ErbB2 reactions are performed in 96-well polystyrene round-bottomed plates in a final volume of 45 μL. Reaction mixtures contain 50 mM 4-morpholinepropanesulfonic acid (pH 7.5), 2 mM MnCl2, 10 μM ATP, 1 μCi of [γ33P] ATP/reaction, 50 μM Peptide A [Biotin-(amino hexonoic acid)-EEEEYFELVAKKK-CONH2], 1 mM dithiothreitol, and 1 μL of DMSO containing serial dilutions of this compound beginning at 10 μM. The reaction is initiated by adding the indicated purified type-1 receptor intracellular domain. The amount of enzyme added is 1 pmol/reaction (20 nM). Reactions are terminated after 10 minutes at 23°C by adding 45 μL of 0.5% phosphoric acid in water. The terminated reaction mix (75 μL) is transferred to phosphocellulose filter plates. The plates are filtered and washed three times with 200 μL of 0.5% phosphoric acid. Scintillation cocktail (50 μL) is added to each well, and the assay is quantified by counting in a Packard Topcount. IC50 values are generated from 10-point dose-response curves.
|
|
| In vivo |
Oral administration of Lapatinib (~100 mg/kg) twice daily significantly inhibits the growth of BT474 and HN5 xenografts in a dose-dependent manner.
|
References |
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | ERBB2 / pERBB2 / p53 / Mdm2 / MdmX / pERK / Hsp70 pEGFR / EGFR / pAkt / Akt / pmTOR / mTOR / PARP / c-PARP p-HER2 / HER2 / p-HER3 / HER3 / p-S6 / S6 / p-4EBP1 |
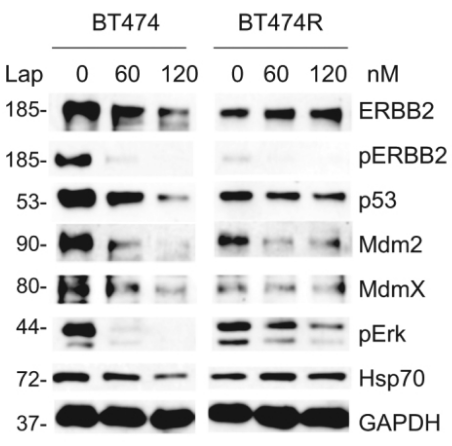
|
29799521 |
| Immunofluorescence | LC3 Vimentin / E-cadherin |
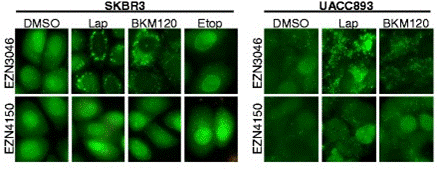
|
26637440 |
| Growth inhibition assay | Cell viability (OE19) Cell viability (A431 cells) |
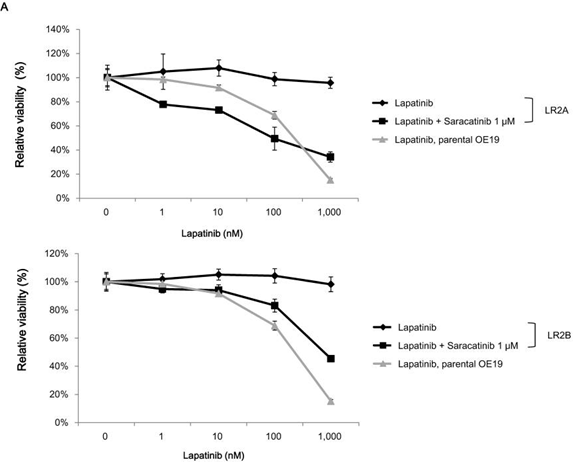
|
25350844 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT00455039 | Withdrawn | Breast Cancer |
University of New Mexico |
July 31 2023 | Phase 1|Phase 2 |
| NCT04608409 | Active not recruiting | Ovarian Cancer |
Frederick R. Ueland M.D.|National Cancer Institute (NCI)|University of Kentucky |
March 17 2021 | Phase 1 |
| NCT03075995 | Unknown status | Breast Cancer |
Sun Yat-sen University |
April 12 2017 | Not Applicable |
| NCT02338245 | Completed | Metastatic Breast Cancer |
ASLAN Pharmaceuticals |
December 29 2014 | Phase 2 |
| NCT02294786 | Terminated | Cancer |
Novartis Pharmaceuticals|Novartis |
December 17 2014 | Phase 2 |
| NCT02158507 | Active not recruiting | Metastatic Triple Negative Breast Cancer |
University of Alabama at Birmingham|Breast Cancer Research Foundation of Alabama|GlaxoSmithKline|AbbVie |
September 2014 | Not Applicable |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
Frequently Asked Questions
Question 1:
If I want to use this compound via injection in tumor-bearing mice, how could I prepare the solution?
Answer:
For I.P. administration, the compound solution should be clear solution. It can be dissolved in 2% DMSO/30% PEG 300/5% Tween 80/ddH2O at 10 mg/ml for clear solution.






































