- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Isavuconazole Fungal inhibitor
Cat.No.S3722
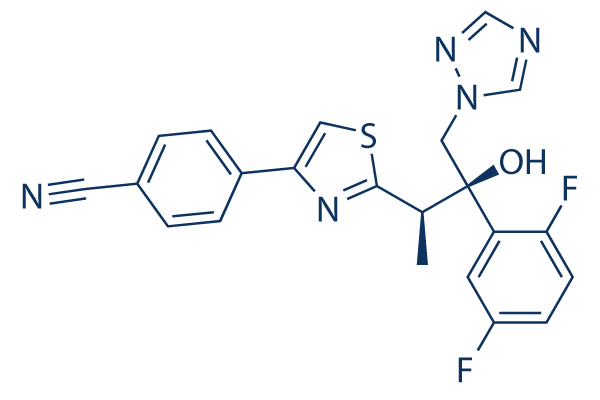
Chemical Structure
Molecular Weight: 437.47
Jump to
Quality Control
| Related Targets | Integrase Bacterial Antibiotics Anti-infection Antiviral COVID-19 Parasite Reverse Transcriptase HIV HCV Protease |
|---|---|
| Other Fungal Inhibitors | Cycloheximide Tolnaftate Neticonazole Amorolfine HCl Pseudolaric Acid B Butylparaben Climbazole Thimerosal Neticonazole Hydrochloride Juglone |
Solubility
|
In vitro |
DMSO
: 87 mg/mL
(198.87 mM)
Ethanol : 87 mg/mL Water : Insoluble |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 437.47 | Formula | C22H17F2N5OS |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 241479-67-4 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | BAL-4815, RO-0094815 | Smiles | CC(C1=NC(=CS1)C2=CC=C(C=C2)C#N)C(CN3C=NC=N3)(C4=C(C=CC(=C4)F)F)O | ||
Mechanism of Action
| In vitro |
Isavuconazole inhibits cytochrome P450 (CYP)–dependent 14α-lanosterol demethylation, which is essential for fungal cell membrane ergosterol synthesis. This blockade produces methylated sterols in the fungal membrane, altering its function and allowing the accumulation of ergosterol toxic precursors in the cytoplasm, which leads to cell death.
|
|---|---|
| In vivo |
Isavuconazonium sulfate, the prodrug of isavuconazole, is available in both intravenous and oral formulations. After intravenous infusion, the prodrug is broken down quickly to the active component, isavuconazole, and an inactive cleavage product. Following oral administration, plasma concentrations of the active compound reach maximum concentrations (Cmax) by 2–3 hours; the prodrug and cleavage product are not measurable in plasma after oral administration. In healthy adult volunteers, isavuconazole exhibits linear and dose-proportional pharmacokinetics. The oral bioavailability of isavuconazole is 98%. Absorption of isavuconazole is not affected by food intake. In addition to excellent bioavailability, isavuconazole serum concentrations show low intersubject variability. In healthy volunteers, the Cmax at steady state was 2.5 ± 1.0 µg/mL. Isavuconazole has a large volume of distribution, is >99% protein bound, and has a long terminal half-life of 100-130 hours. Metabolism of isavuconazole takes place in the liver via the CYP enzyme family, specifically CYP3A4 and CYP3A5 isoenzymes.
|
References |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05630976 | Recruiting | Invasive Fungal Disease |
Pfizer |
February 7 2023 | Phase 4 |
| NCT04486885 | Unknown status | Cerebral Aspergillosis|Invasive Aspergillosis |
Imagine Institute |
August 1 2021 | -- |
| NCT04777058 | Completed | Invasive Fungal Infections|Pharmacokinetics|Intensive Care Unit |
Radboud University Medical Center |
March 1 2021 | -- |
| NCT04550936 | Completed | Invasive Aspergillosis|Mucormycosis |
Pfizer |
March 10 2021 | -- |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































