- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Cordycepin MMP inhibitor
Cat.No.S3610
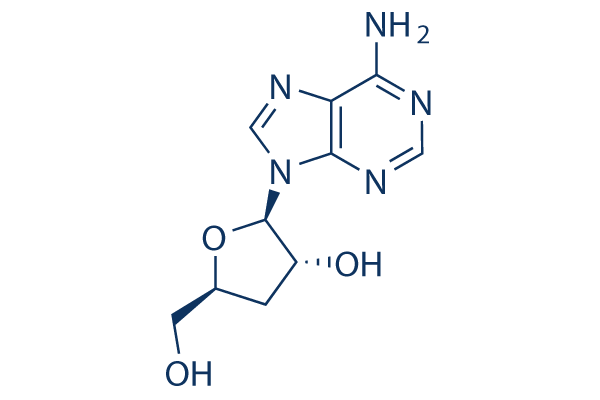
Chemical Structure
Molecular Weight: 251.24
Jump to
Quality Control
Batch:
Purity:
99.97%
99.97
Solubility
|
In vitro |
DMSO
: 50 mg/mL
(199.01 mM)
Water : 25 mg/mL Ethanol : 1 mg/mL |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 251.24 | Formula | C10H13N5O3 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 73-03-0 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | 3'-Deoxyadenosine | Smiles | C1C(OC(C1O)N2C=NC3=C(N=CN=C32)N)CO | ||
Mechanism of Action
| In vitro |
Cordycepin increases interleukin (IL)-10 expression, decreased IL-2 expression and suppresses T lymphocyte activity. It also up-regulates IL-1beta, IL-6, IL-8 and TNF-alpha and suppresses phytohemagglutinin (PHA)-induced production of IL-2, IL-4, IL-5, IFN-gamma and IL-12. The structure of this compound is very much similar with cellular nucleoside, adenosine and acts like a nucleoside analogue. It lacks 3' hydroxyl group in its structure. This compound provokes RNA chain termination and interferes in mTOR signal transduction. At higher doses, it inhibits cell attachment and reduces focal adhesion. under low nutritional stress, this chemical activates AMPK which blocks the activity of mTORC1 and mTORC2 complex. The inactivated mTORC2 complex cannot activate AKT 1 kinase fully, which in turn blocks mTOR signal transduction inhibiting translation and further cell proliferation and growth. It also induces apoptosis by enhancing JNK and p38 kinase activity and increasing the protein expression of Bcl-2 pro-apoptotic molecules. This compound has anti-tumor effect on mouse melanoma and lung carcinoma cells and human oral cancer cells.
|
|---|---|
| In vivo |
Orally administered cordycepin inhibits melanoma cell growth in mice with no adverse effects. Oral administration of this compound at dose of 10 mg/kg significantly improves Y-maze learning performance both in healthy and ischemic mice. However, it at dose of 5 mg/kg enhanced Y-maze learning only in ischemic mice but not healthy mice. This chemical significantly decreases the neuronal loss induced by ischemia in hippocampal CA1 and CA3 regions.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT00709215 | Unknown status | Refractory TdT-Positive Leukemia |
OncoVista Inc.|AAIPharma |
June 2008 | Phase 1|Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































