research use only
GSK1265744 (Cabotegravir) HIV Integrase inhibitor
Cat.No.S7766
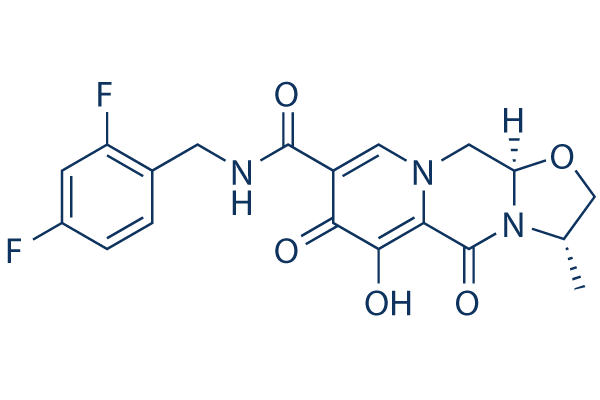
Chemical Structure
Molecular Weight: 405.35
Quality Control
| Related Targets | Bacterial Antibiotics Anti-infection Fungal Antiviral COVID-19 Parasite Reverse Transcriptase HIV HCV Protease |
|---|---|
| Other Integrase Inhibitors | MK-2048 BMS-707035 Robinetin Lavendustin B |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| HEK293T | Antiviral assay | 2 days | Antiviral activity against pseudo Human immunodeficiency virus infected in HEK293T cells after 2 days, IC50 = 0.0005 μM. | 23845180 | ||
| MT4 | Antiviral assay | 4 to 5 days | Antiviral activity against Human immunodeficiency virus 1 3B infected in human MT4 cells after 4 to 5 days by bioluminescence assay, IC50 = 0.0013 μM. | 23845180 | ||
| HEK293T | Antiviral assay | 2 days | Antiviral activity against pseudo Human immunodeficiency virus infected in HEK293T cells after 2 days in presence of human serum albumin, IC50 = 0.03 μM. | 23845180 | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 30 mg/mL
(74.01 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 405.35 | Formula | C19H17F2N3O5 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1051375-10-0 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | GSK744, S/GSK1265744 | Smiles | CC1COC2N1C(=O)C3=C(C(=O)C(=CN3C2)C(=O)NCC4=C(C=C(C=C4)F)F)O | ||
Mechanism of Action
| Targets/IC50/Ki |
HIV integrase
|
|---|---|
| In vitro |
Cabotegravir (GSK1265744) inhibits HIV replication with EC50 of 0.22 nM and 0.34-1.3 nM against HIV-1 Ba-L and NL432, respectively. It produces cytotoxicity with CC50 of 6.4, 5.0, 9.2, and 13 μM in proliferating IM-9, U-937, MT-4, and Molt-4 cell lines, respectively.
|
| Kinase Assay |
In vitro strand transfer assay
|
|
The inhibitory concentrations of Cabotegravir (GSK1265744) are measured in a strand transfer assay using recombinant HIV IN. A complex of integrase and biotinylated donor DNA–streptavidin-coated SPA beads is formed by incubating 2 μM purified recombinant integrase with 0.66 μM biotinylated donor DNA–4 mg/mL streptavidin-coated SPA beads in 25 mM sodium MOPS pH 7.2, 23 mM NaCl, and 10 mM MgCl2 for 5 minutes at 37°C. These beads are spun down and preincubated with diluted INSTIs for 60 minutes at 37°C. Next, [3H]-labeled target DNA substrate is added to give a final concentration of 7 nM substrate, and the strand transfer reaction is incubated at 37°C for 25 to 45 minutes, which allowed for a linear increase in strand transfer of donor DNA to radiolabeled target DNA. The signal is read using a Wallac MicroBeta scintillation plate reader.
|
|
| In vivo |
Cabotegravir (GSK1265744) (50 mg/kg) treatment protects rhesus macaques against three high-dose SHIV challenges.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06319105 | Recruiting | Oral Pre-exposure Prophylaxis (PrEP)|Long-acting Injectable Cabotegravir for PrEP |
Georgetown University|AIDS Vaccine Advocacy Coalition|Centers for Disease Control and Prevention|Center for the Development of People|Cooper/Smith|Elizabeth Glaser Pediatric AIDS Foundation|Family Health Services|Healthqual|Johns Hopkins Research Project Malawi|Johns Hopkins Bloomberg School of Public Health|Kamuzu University of Health Sciences|Blantyre District Health Office Malawi|Lilongwe District Health Office Malawi|Lighthouse Trust|Ministry of Health Malawi|National AIDS Commission Malawi|Pakachere Institute of Health and Development Communication|Partners in Hope|Population Services International|University of North Carolina|United States Agency for International Development (USAID)|United States President''s Emergency Plan for AIDS Relief|Quantitative Engineering Design|Clinton Health Access Initiative-Malawi |
March 26 2024 | -- |
| NCT06104306 | Recruiting | HIV-1-infection |
Gilead Sciences |
December 13 2023 | Phase 4 |
| NCT05986084 | Recruiting | Pre-Exposure Prophylaxis (PrEP)|Breast Feeding |
Beth Israel Deaconess Medical Center|Botswana Harvard AIDS Institute Partnership|Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)|ViiV Healthcare |
November 30 2023 | Phase 4 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































