- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Prazosin HCl Adrenergic Receptor antagonist
Cat.No.S1424
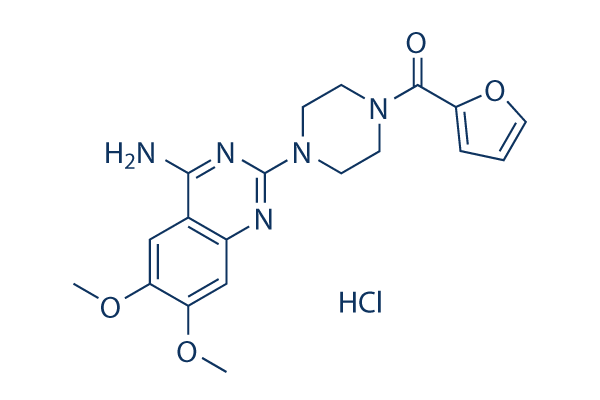
Chemical Structure
Molecular Weight: 419.86
Jump to
Quality Control
Batch:
Purity:
99.99%
99.99
| Related Targets | CXCR Hedgehog/Smoothened PKA AChR 5-HT Receptor Histamine Receptor Dopamine Receptor Ras KRas GPR |
|---|---|
| Other Adrenergic Receptor Inhibitors | Zenidolol (ICI-118551) Hydrochloride Yohimbine HCl L755507 Atipamezole Higenamine hydrochloride Fenoterol hydrobromide Medetomidine HCl Rauwolscine hydrochloride Deoxycorticosterone acetate Detomidine HCl |
Solubility
|
In vitro |
DMSO
: 3 mg/mL
(7.14 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 419.86 | Formula | C19H21N5O4·HCl |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 19237-84-4 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | cp-12299-1 | Smiles | COC1=C(C=C2C(=C1)C(=NC(=N2)N3CCN(CC3)C(=O)C4=CC=CO4)N)OC.Cl | ||
Mechanism of Action
| Targets/IC50/Ki |
alpha-adrenergic receptor
|
|---|---|
| In vitro |
Prazosin leads to a significant increase in VEGF concentration in endothelial cells and angiogenesis only if eNOS is present. Prazosin binds to the α1-adrenergic receptors that are present on smooth muscle cells surrounding all larger blood vessels. Prazosin (0.1 nM) blocks the increases in perfusion pressure caused by electrical stimulation of the perimesenteric nerves but does not significantly reduce the vasomotor effect of exogenous noradrenaline.
|
| In vivo |
Prazosin application leads to the expansion of the capillary system by modulation of the hemodynamic environment (flow rate, shear stress) in skeletal muscle. Prazosin induces angiogenesis in extensor digitorum longus (EDL) muscles of C57BL/6 mice but not eNOS-knockout mice. Prazosin (0.5-2.0 mg/kg) blocks Yohimbine-induced reinstatement of food and alcohol seeking, as well as footshock-induced reinstatement of alcohol seeking in rats. Prazosin (0.2mg kg(-1) s.c.) causes an enhancement of a suppression of conditioned avoidance response in the presence of the dopamine D2 receptor antagonist raclopride (0.05-0.20 mg/kg s.c.) in rats. Prazosin administration alone (1 mg/kg, s.c.) only slightly reduces horizontal activity during an initial 10 min measurement period, although it consistently reduces rearing in freely moving rats. Prazosin effectively suppresses the locomotor stimulation caused by either dose of MK-801 throughout the whole observation period in freely moving rats.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05189977 | Terminated | Post-traumatic Stress Disorder (PTSD) |
Otsuka Pharmaceutical Development & Commercialization Inc.|Iqvia Pty Ltd |
September 7 2022 | Phase 1 |
| NCT04365257 | Terminated | COVID-19 |
Johns Hopkins University|Fast Grants |
May 13 2020 | Phase 2 |
| NCT03045016 | Completed | Acute Stress Disorder |
Hospices Civils de Lyon |
April 21 2017 | Phase 2 |
| NCT02193256 | Completed | Cigarette Smoking|Alcohol Use Disorders |
Yale University|National Institute on Alcohol Abuse and Alcoholism (NIAAA) |
July 2014 | Early Phase 1 |
| NCT01178138 | Completed | Continuous Methamphetamine Abuse |
University of Arkansas|National Institute on Drug Abuse (NIDA) |
December 2009 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































