research use only
Irbesartan Angiotensin Receptor antagonist
Cat.No.S1507
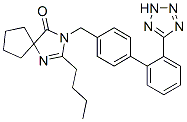
Chemical Structure
Molecular Weight: 428.53
Quality Control
| Related Targets | CXCR Hedgehog/Smoothened PKA Adrenergic Receptor AChR 5-HT Receptor Histamine Receptor Dopamine Receptor Ras KRas |
|---|---|
| Other Angiotensin Receptor Inhibitors | PD123319 ML221 A-779 Fimasartan Olodanrigan (EMA401) Buloxibutid AVE 0991 |
Solubility
|
In vitro |
DMSO
: 1 mg/mL
(2.33 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 428.53 | Formula | C25H28N6O |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 138402-11-6 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | BMS-186295, SR-47436 | Smiles | CCCCC1=NC2(CCCC2)C(=O)N1CC3=CC=C(C=C3)C4=CC=CC=C4C5=NNN=N5 | ||
Mechanism of Action
| Features |
Irbesartan is a longer acting AT1 receptor antagonist relative to losartan and valsartan.
|
|---|---|
| Targets/IC50/Ki |
AT1 receptor
1.3 nM
|
| In vitro |
Irbesartan competes with angiotensin II (AII) for binding at the AT1 receptor subtype and antagonizes AII-induced contraction in rabbit aorta ring with IC50 of 4 nM. This compound has no affinity for AT2 receptors. It (10 μM) blocks angiotensin II induced increase in αv, β1, β3, and β5 integrins, osteopontin, and α-actinin mRNA and protein levels in rat cardiac fibroblasts, leading to the decrease of cell attachment to extracellular matrix (ECM) proteins. This chemical treatment markedly induces the expression of the adipogenic marker gene adipose protein 2 (aP2) in 3T3-L1 cells in a concentration-dependent manner with EC50 of 3.5 μM and 3.3-fold induction at the concentration of 10 μM. It (10 μM) markedly induces transcriptional activity of the peroxisome proliferator–activated receptor-γ (PPARγ) by 3.4-fold independent of its AT1 receptor blocking action. Pretreatment with this compound (~10 μM) decreases angiotensin II-induced apoptosis in rat vascular smooth muscle cells by blocking angiotensin II internalization in a concentrationdependent manner. |
| In vivo |
Oral administration of Irbesartan (1 mg/kg) reduces angiotensin II (AII)-induced hypertension, equipotent with losartan in conscious normotensive rats, markedly more active than losartan (10 mg/kg) in normotensive cynomolgus monkeys. Administration of this compound (7 mg/kg/day) significantly prevents skeletal muscle apoptosis and muscle atrophy in rats with monocrotaline-induced congestive heart failure (CHF), which is involved with the decrease of TNFα level and attributed to AT1 receptor blocking. |
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06256991 | Not yet recruiting | Chronic Kidney Diseases|Hyperkalemia|Hypertension |
University Medical Center Groningen|Amsterdam University Medical Center|Medical Centre Leeuwarden|Isala|Vifor Pharma Inc.|Dutch Kidney Foundation|Health Holland |
April 2024 | Phase 4 |
| NCT06208072 | Recruiting | Primary Hypertension|Obesity |
Hippocration General Hospital|National and Kapodistrian University of Athens |
September 1 2023 | Not Applicable |
| NCT04983979 | Terminated | CKD|Diabetes Mellitus Type 2|Hyperkalemia |
Barts & The London NHS Trust |
June 17 2022 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































