- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
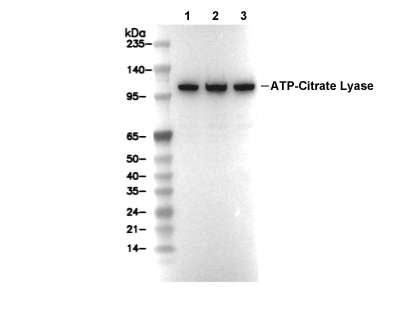
ATP citrate lyase Antibody [N17N8]
Cat.No.: F4744
Application:
Reactivity:
Usage Information
| Dilution |
|---|
|
| Application |
|---|
| WB |
| Reactivity |
|---|
| Human, Mouse, Rat |
| Source |
|---|
| Rabbit Monoclonal Antibody |
| Storage Buffer |
|---|
| PBS, pH 7.2+50% Glycerol+0.05% BSA+0.01% NaN3 |
| Storage (from the date of receipt) |
|---|
| -20°C (avoid freeze-thaw cycles), 2 years |
| Predicted MW |
|---|
| 125 kDa |
Biological Description
| Specificity |
|---|
| ATP citrate lyase Antibody [N17N8] detects endogenous levels of total ATP citrate lyase protein. |
| Clone |
|---|
| N17N8 |
| Synonym(s) |
|---|
| ACLY; ATP-citrate synthase; EC:2.3.3.8; ATP-citrate (pro-S-)-lyase (ACL); Citrate cleavage enzyme |
| Background |
|---|
| ATP citrate lyase (ACLY), a cytosolic homotetrameric enzyme at the interface of glucose and lipid metabolism, cleaves citrate derived from mitochondrial export into acetyl-CoA and oxaloacetate to fuel de novo lipogenesis, cholesterol synthesis, and histone acetylation, organized into two major domains per subunit, an N-terminal citrate synthase-homology (CSH) domain encompassing residues ~1-700 that binds citrate and CoA to form citryl-CoA via His760 loop swinging and pantothenate arm translocation, coordinated with a C-terminal ATP-grasp citrate lyase-like (CL) domain (~700-1105) housing the nucleotide-binding site where Mg²⁺-ATP phosphorylation occurs through Asp938 and Lys828 stabilization of the γ-phosphate transfer in a sequential ordered Bi Bi mechanism, breaking D2 symmetry across subunits for energetic coupling during conformational shifts from apo (open CCS) to compact intermediate states with citryl-1-phosphate and CoA at the CCS catalytic center (G281-283, S308, E599, G664-665). ACLY drives anabolic pathways by delivering acetyl-CoA for fatty acid synthase and HMGCR in nutrient-rich conditions via SREBP-1/2 activation, undergoes Akt-mediated Ser455 phosphorylation to relieve citrate allostery and boost Vmax by 3-fold while favoring ATP over CTP, couples with ACSS2 for acetate salvage during hypoxia/glucose restriction to sustain proliferation, and interfaces with epigenetics through nuclear translocation for H3K27ac at promoters of MYC/IGF2 in cancer cells, with subunit conformational barriers (His760 loops, radius of gyration drop at catalytic center formation) synchronized to biochemical steps, citrate phosphorylation, citryl-CoA formation, and cleavage, enabling ~10⁶-fold rate acceleration. Dysregulated ACLY overexpression fuels metabolic reprogramming in tumors (breast, prostate, glioblastoma) via PI3K/AKT/mTORC1 hyperactivation, promotes hepatic steatosis under high-fructose diets through ChREBP induction, and its inhibition by bempedoic acid or ND-630 reduces LDL-C and tumor growth by starving lipogenesis without impairing ketogenesis. |
| References |
|---|
|
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.