research use only
Urethane Bacterial inhibitor
Cat.No.S4544
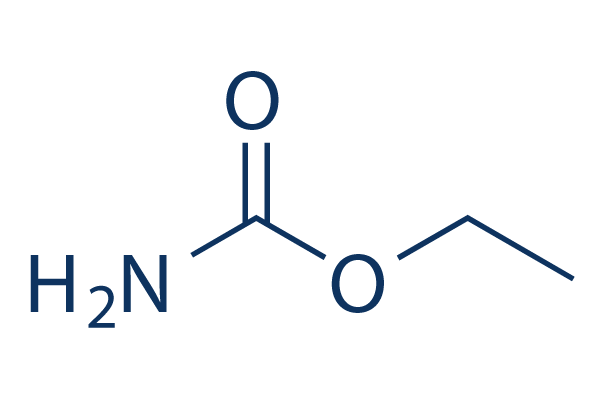
Chemical Structure
Molecular Weight: 89.09
| Related Targets | Integrase Antibiotics Anti-infection Fungal Antiviral COVID-19 Parasite Reverse Transcriptase HIV HCV Protease |
|---|---|
| Other Bacterial Inhibitors | Berberine BTZ043 Racemate Teicoplanin Pefloxacin Mesylate Furagin Ornidazole Proanthocyanidins Solithromycin Skatole Berberine Sulfate |
Solubility
|
In vitro |
DMSO
: 17 mg/mL
(190.81 mM)
Water : 17 mg/mL Ethanol : 17 mg/mL |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 89.09 | Formula | C3H7NO2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 51-79-6 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | Carbamic acid ethyl ester, Ethyl carbamate, Ethylurethane | Smiles | CCOC(=O)N | ||
Mechanism of Action
| Targets/IC50/Ki |
AMPA receptor
34 mM(EC50)
glycine receptors
46 mM(EC50)
GABAA receptors
64 mM(EC50)
NMDA receptors
70 mM(EC50)
α4β2 nACh receptor
114 mM(EC50)
|
|---|---|
| In vitro |
urethane has a spectrum of action on ion channels, which is distinct from other anesthetics. It significantly potentiates the current responses of both GABAA and glycine receptors in a reversible and concentration-dependent manner. Conversely, this compound (10–300 mM) inhibits the responses of NMDA and AMPA receptors. Also, it potentiates the function of an nACh receptor and neuronal nicotinic acetylcholine, γ-aminobutyric acid A, and glycine receptors.
|
| In vivo |
Urethane, a carcinogenic substance, is favored for acute in vivo electrophysiological experiments because it induces long-lasting steady level of anesthesia with muscle relaxation and minimally affects the autonomic and cardiovascular systems. This compound affects both inhibitory and excitatory systems but the magnitude of the alterations is less than that produced by other more selective anesthetics. But also, this chemical is usually restricted to terminal (acute) experiments due to its potential long-term toxicity.
|
References |
|
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































