research use only
Miltefosine Akt inhibitor
Cat.No.S3056
Chemical Structure
Molecular Weight: 407.57
Quality Control
| Related Targets | PI3K mTOR GSK-3 ATM/ATR DNA-PK AMPK PDPK1 PTEN PP2A PDK |
|---|---|
| Other Akt Inhibitors | SC79 Ipatasertib (GDC-0068) MK-2206 Dihydrochloride Perifosine AZD5363 (Capivasertib) GSK690693 Triciribine (API-2) CCT128930 Afuresertib (GSK2110183) A-674563 HCl |
Solubility
|
In vitro |
Water : 82 mg/mL Ethanol : 82 mg/mL
DMSO
: Insoluble
|
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 407.57 | Formula | C21H46NO4P |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 58066-85-6 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | Hexadecylphosphocholine | Smiles | CCCCCCCCCCCCCCCCOP(=O)([O-])OCC[N+](C)(C)C | ||
Mechanism of Action
| Targets/IC50/Ki |
Akt
PI3K
PKC
~7 μM
|
|---|---|
| In vitro |
Miltefosine is an alkylphosphocholine drug with demonstrated activity against various parasite species and cancer cells as well as some pathogenic bacteria and fungi. This compound inhibits PKC from NIH3T3 cells in cell-free extracts with a IC50 of about 7 μM. It targets HIV infected macrophages, which play a role in vivo as long-lived HIV-1 reservoirs. This chemical acts by inhibiting the PI3K/Akt pathway, thus removing the infected macrophages from circulation, without affecting healthy cells. It inhibits the PI3K/Akt survival pathway in carcinoma cell lines. This compound causes skeletal muscle insulin resistance in vitro by interfering with the insulinsignalling pathway and inhibiting insulin-stimulated glucose
uptake. It inhibits insulin-stimulated Akt phosphorylation in
a dose-dependent manner with 75% inhibition at 40 μM and 98% inhibition at 60 μM.
|
| In vivo |
Miltefosine inhibits anti-IgE induced histamine release from human skin mast cells. This compound can reduce cytokines IL-1β, IL-4, and IL-6 in certain skin tissue cells and also strongly impede the esterification of cholesterol.
|
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
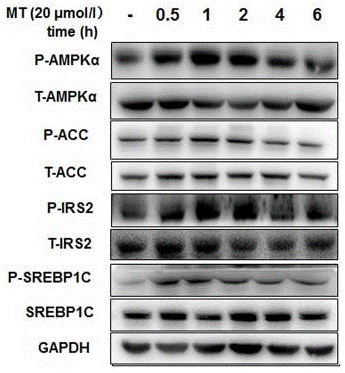
| Western blot | p-AMPKα / AMPKα / p-ACC / ACC / p-IRS2 / IRS2 / p-SREBP1C / SREBP1C p-AKT / AKT / VEGF / MMP-9 / XIAP / Cyclin D1 / MCL-1 / C-FLIP |
|
27681040 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05493059 | Not yet recruiting | Cutaneous Leishmaniases|Treatment Adherence|Primary Health Care|Drug Evaluation |
Centre Hospitalier de Cayenne |
August 8 2022 | -- |
| NCT03399955 | Unknown status | PKDL - Post-Kala-Azar Dermal Leishmanioid |
Drugs for Neglected Diseases |
May 9 2018 | Phase 2 |
| NCT02193022 | Completed | Post Kala Azar Dermal Leishmaniasis |
International Centre for Diarrhoeal Disease Research Bangladesh|Thrasher Research Fund |
July 2014 | Phase 3 |
| NCT01462500 | Completed | Cutaneous Leishmaniasis |
Centro Internacional de Entrenamiento e Investigaciones Médicas|Academisch Medisch Centrum - Universiteit van Amsterdam (AMC-UvA) |
October 2011 | Phase 4 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































