research use only
Desvenlafaxine Serotonin Transporter inhibitor
Cat.No.S4113
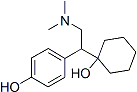
Chemical Structure
Molecular Weight: 263.38
Quality Control
| Related Targets | Adrenergic Receptor AChR 5-HT Receptor COX Calcium Channel Histamine Receptor Dopamine Receptor GABA Receptor TRP Channel Cholinesterase (ChE) |
|---|---|
| Other Serotonin Transporter Inhibitors | Dapoxetine |
Solubility
|
In vitro |
DMSO
: 37 mg/mL
(140.48 mM)
Ethanol : 3 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 263.38 | Formula | C16H25NO2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 93413-62-8 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | WY 45233 Succinate | Smiles | CN(C)CC(C1=CC=C(C=C1)O)C2(CCCCC2)O | ||
Mechanism of Action
| Features |
Desvenlafaxine is a major active metabolite of venlafaxine.
|
|---|---|
| Targets/IC50/Ki |
5-HT
40.2 nM(Ki)
Norepinephrine (NE)
558.4 nM(Ki)
|
| In vitro |
Desvenlafaxine is a serotonin-norepinephrine reuptake inhibitor and is the active metabolite of the antidepressant venlafaxine. Similar to venlafaxine, this compound inhibits the neuronal uptake of serotonin and norepinephrine. It shows weak binding affinity (62% inhibition at 100 μM) at the human dopamine (DA) transporter. This chemical inhibits [3H]5-HT or [3H]NE uptake for the hSERT or hNET with IC50 of 47.3 and 531.3 nM, respectively. It has the potential to inhibit CYP2D6, which could result in increased concentrations of drugs metabolized through this pathway. Induction of CYP3A4 is also possible with this agent, which could impact the metabolism of drugs metabolized via this enzyme.
|
| In vivo |
Desvenlafaxine rapidly penetrates the male rat brain and hypothalamus. This compound significantly increases extracellular NE levels compared with baseline in the male rat hypothalamus but had no effect on DA levels using microdialysis. It exhibits a linear and dose-proportional pharmacokinetic single-dose profile in a dose range from 100 to 600 mg/day. The absolute bioavailability of the oral formulation is 80.5%.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT02637193 | Completed | Healthy Subjects |
Pfizer''s Upjohn has merged with Mylan to form Viatris Inc.|Pfizer |
December 2015 | Phase 1 |
| NCT02200406 | Completed | Depression|Opioid Dependence|Methadone Treatment |
Centre hospitalier de l''Université de Montréal (CHUM)|Pfizer |
July 2014 | Phase 4 |
| NCT00888862 | Unknown status | Major Depressive Disorder|Menopausal Staging and Vasomotor Symptoms (for Females) |
Hamilton Health Sciences Corporation|Wyeth is now a wholly owned subsidiary of Pfizer|St. Joseph''s Healthcare Hamilton|McMaster University |
June 2009 | Phase 3 |
| NCT00818155 | Completed | Healthy |
Wyeth is now a wholly owned subsidiary of Pfizer |
January 2009 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































