research use only
Benfotiamine NF-κB inhibitor
Cat.No.S4798
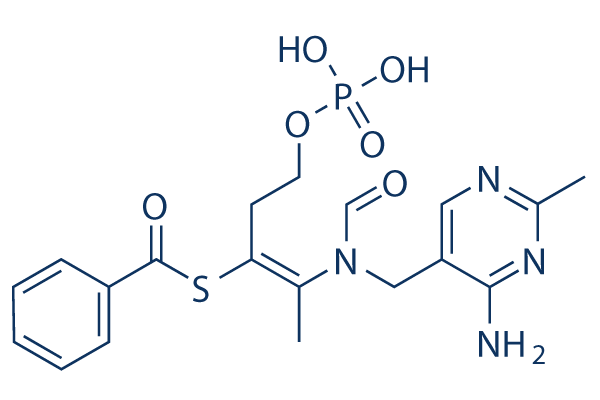
Chemical Structure
Molecular Weight: 466.45
Quality Control
| Related Targets | HDAC Antioxidant ROS IκB/IKK Nrf2 AP-1 MALT NOD |
|---|---|
| Other NF-κB Inhibitors | DCZ0415 Omaveloxolone (RTA-408) BAY 11-7082 (BAY 11-7821) JSH-23 QNZ (EVP4593) Caffeic Acid Phenethyl Ester SC75741 DHA (Dihydroartemisinin) Withaferin A (WFA) Andrographolide |
Solubility
|
In vitro |
5%TFA : 6 mg/mL |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 466.45 | Formula | C19H23N4O6PS |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 22457-89-2 | -- | Storage of Stock Solutions |
|
|
| Synonyms | S-Benzoylthiamine O-monophosphate, Benzoylthiamine monophosphate | Smiles | CC1=NC=C(C(=N1)N)CN(C=O)C(=C(CCOP(=O)(O)O)SC(=O)C2=CC=CC=C2)C | ||
Mechanism of Action
| Targets/IC50/Ki |
NF-κB
|
|---|---|
| In vitro |
Benfotiamine improves the expression of endothelial cell markers in EPCs, restores eNOS levels, and recovers the ability of EPCs to participate in angiogenic processes. It is able to dampen glucose toxicity effects on endothelial progenitors. This compound possesses antitumor activity against leukemia cells. In a panel of nine myeloid leukemia cell lines this chemical impairs the viability of HL-60, NB4, K562 and KG1 cells and also inhibits the growing of primary leukemic blasts. The antitumor activity of this compound is not mediated by apoptosis, necrosis or autophagy, but rather occurs though paraptosis cell death induction. It inhibits the activity of constitutively active ERK1/2 and concomitantly increases the phosphorylation of JNK1/2 kinase in leukemic cells. In addition, this chemical induces the down regulation of the cell cycle regulator CDK3 which results in G1 cell cycle arrest in the sensitive leukemic cells. |
| In vivo |
Benfotiamine might exert vascular and renal benefits by modulating mechanisms independent or downstream of ROS formation. This compound aids the post-ischaemic healing of diabetic animals via PKB/Akt-mediated potentiation of angiogenesis and inhibition of apoptosis. |
References |
|
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































