- Bioactive Compounds
- By Signaling Pathways
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- DNA Damage/DNA Repair
- Compound Libraries
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-Ⅱ
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Plant Extract Library
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at info@selleckchem.com to customize your library.
You could select:
- Antibodies
- Bioreagents
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- Protein Assay
- Protein A/G Magnetic Beads for IP
- Anti-Flag magnetic beads
- Anti-Flag Affinity Gel
- Anti-Myc magnetic beads
- Anti-HA magnetic beads
- Poly DYKDDDDK Tag Peptide lyophilized powder
- Protease Inhibitor Cocktail
- Protease Inhibitor Cocktail (EDTA-Free, 100X in DMSO)
- Phosphatase Inhibitor Cocktail (2 Tubes, 100X)
- Cell Biology
- Cell Counting Kit-8 (CCK-8)
- Animal Experiment
- Mouse Direct PCR Kit (For Genotyping)
- New Products
- Contact Us
research use only
5-Fluorouracil (5-FU) DNA/RNA Synthesis inhibitor
5-FU (5-Fluorouracil) is a DNA/RNA synthesis inhibitor, which interrupts nucleotide synthetic by inhibiting thymidylate synthase (TS) in tumor cells. Fluorouracil induces apoptosis and can be used in the treatment of HIV.
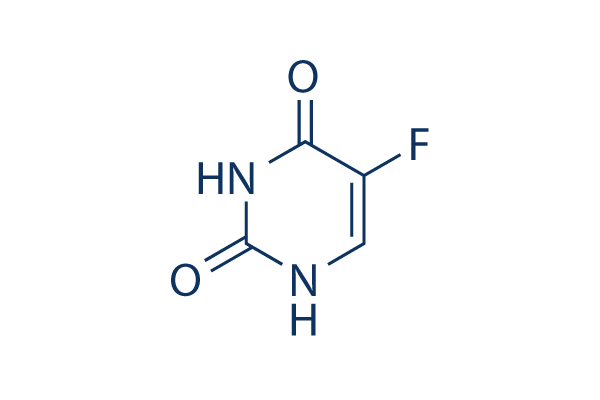
Chemical Structure
Molecular Weight: 130.08
Purity & Quality Control
Batch:
Purity:
99.97%
99.97
Related Products
| Related Targets | tRNA synthetase RdRp DNA synthesis helicase ribonucleotide reductase | Click to Expand |
|---|---|---|
| Related Products | CX-5461 (Pidnarulex) SCR7 Favipiravir (T-705) RK-33 Carmofur Triapine (3-AP) B02 BMH-21 YK-4-279 Bergapten Tegafur (FT-207) Azaguanine-8 Halofuginone Adenine HCl Mizoribine Cyclocytidine HCl Thymidine APX-3330 Nedaplatin 6-Thio-dG | Click to Expand |
| Related Compound Libraries | FDA-approved Drug Library Natural Product Library Apoptosis Compound Library DNA Damage/DNA Repair compound Library Cell Cycle compound library | Click to Expand |
Signaling Pathway
Cell Culture and Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| NCI-H292 | Growth Inhibition Assay | 72 h | IC50> 25 μg/mL | 24095176 | ||
| HL-60 | Growth Inhibition Assay | 72 h | IC50=8.601 μg/mL | 24095176 | ||
| HT-29 | Growth Inhibition Assay | 72 h | IC50> 25 μg/mL | 24095176 | ||
| MCF-7 | Growth Inhibition Assay | 72 h | IC50=20 μg/mL | 24095176 | ||
| L1210/0 | Function assay | Effect on L1210/0 cells in culture, IC50 = 0.3 μM. | 2362278 | |||
| CCRF-CEM | Function assay | Effect on CCRF-CEM cells in culture, IC50 = 2 μM. | 2362278 | |||
| P388 | Antiproliferative assay | 48 hrs | Antiproliferative activity against mouse P388 cells after 48 hrs by MTT assay, IC50 = 0.609 μM. | 2778449 | ||
| HT-29 | Antiproliferative assay | 96 hrs | Antiproliferative activity against human HT-29 cells after 96 hrs by MTT assay, IC50 = 0.69 μM. | 2778449 | ||
| L1210 | Growth inhibition assay | Inhibition of cell growth of L1210 mouse Leukemia cells, IC50 = 0.3 μM. | 2967375 | |||
| L1210 | Growth inhibition assay | Concentration required for the 50% inhibition of growth of L1210 mouse leukemia cells in culture, IC50 = 0.5 μM. | 7120290 | |||
| CCRF-CEM | Growth inhibition assay | Concentration required for the 50% inhibition of growth of CCRF-CEM human leukemia cells in culture, IC50 = 5 μM. | 7120290 | |||
| L1210 | Growth inhibition assay | 48 hrs | In vitro growth inhibition of L1210 cells 48 hours after administration of compound, IC50 = 0.12 μM. | 7629806 | ||
| L1210 | Growth inhibition assay | 24 hrs | In vitro growth inhibition of L1210 cells 24 hours after administration of compound, IC50 = 0.22 μM. | 7629806 | ||
| L1210 | Growth inhibition assay | 8 hrs | In vitro growth inhibition of L1210 cells 8 hours after administration of compound, IC50 = 0.63 μM. | 7629806 | ||
| L1210 | Growth inhibition assay | 2 hrs | In vitro growth inhibition of L1210 cells 2 hours after administration of compound, IC50 = 2.2 μM. | 7629806 | ||
| CHO | Growth inhibition assay | Growth inhibitory activity against CHO cells in culture., IC50 = 5 μM. | 7853342 | |||
| SCLC | Cytotoxicity assay | Cytotoxicity against human SCLC cells resistant to cisplatin (SCLC/CDDP), IC50 = 8.4 μM. | 8258835 | |||
| T-24 | Cytotoxicity assay | Tested for cytotoxicity against antigen negative T-24 bladder carcinoma cells having only 3-5% antigen expression, IC50 = 4.1 μM. | 8496926 | |||
| 791T | Cytotoxicity assay | Tested for cytotoxicity against antigen positive 791T osteosarcoma cells having only 3-5% antigen expression, IC50 = 5.5 μM. | 8496926 | |||
| 9L | Anticancer assay | Tested in vitro for anticancer activity against 9L cells, IC50 = 1 μM. | 10072683 | |||
| SK-MEL-28 | Anticancer assay | Tested in vitro for anticancer activity against SK-MEL-28 cells, IC50 = 4.5 μM. | 10072683 | |||
| PBM | Cytotoxicity assay | Tested in vitro for cytotoxicity against PBM cells, IC50 = 5 μM. | 10072683 | |||
| Vero | Cytotoxicity assay | Tested in vitro for cytotoxicity against Vero cells, IC50 = 6.81 μM. | 10072683 | |||
| SK-MES-1 | Anticancer assay | Tested in vitro for anticancer activity against SK-MES-1 cells, IC50 = 13.1 μM. | 10072683 | |||
| HepG2 | Anticancer assay | Tested in vitro for anticancer activity against HepG2 cells, IC50 = 37.9 μM. | 10072683 | |||
| MCF-7 | Anticancer assay | Tested in vitro for anticancer activity against MCF-7 cells, IC50 = 41 μM. | 10072683 | |||
| L1210 | Cytotoxicity assay | In vitro cytotoxicity against murine Leukemia L1210 cells., IC50 = 0.62 μM. | 10229634 | |||
| KB | Cytotoxicity assay | In vitro cytotoxicity against human oral epidermoid carcinoma KB cells., IC50 = 2.3 μM. | 10229634 | |||
| L1210 | Growth inhibition assay | Concentration required to inhibit 50% of cell growth on murine L1210 cells., IC50 = 0.28 μM. | 11052798 | |||
| P388 D1 | Growth inhibition assay | Concentration required to inhibit 50% of cell growth on murine P388 D1 cells., IC50 = 0.49 μM. | 11052798 | |||
| Molt 4/C8 | Growth inhibition assay | Concentration required to inhibit 50% of cell growth on human Molt 4/C8 cells., IC50 = 23 μM. | 11052798 | |||
| L1210 | Growth inhibition assay | 48 hrs | Growth inhibition in L1210 mouse Leukemia cells for a treatment time of 48 hr, IC50 = 0.125 μM. | 11063625 | ||
| L1210 | Growth inhibition assay | 24 hrs | Growth inhibition in L1210 mouse Leukemia cells for a treatment time of 24 hr, IC50 = 0.22 μM. | 11063625 | ||
| L1210 | Growth inhibition assay | 8 hrs | Growth inhibition in L1210 mouse Leukemia cells for a treatment time of 8 hr, IC50 = 0.63 μM. | 11063625 | ||
| L1210 | Growth inhibition assay | 2 hrs | Growth inhibition in L1210 mouse Leukemia cells for a treatment time of 2 hr, IC50 = 2.2 μM. | 11063625 | ||
| Colon 26-L5 | Antiproliferative assay | 72 hrs | Antiproliferative activity against mouse Colon 26-L5 cells assessed as cell viability after 72 hrs by MTT assay, IC50 = 0.5 μM. | 11575942 | ||
| B16-BL6 | Antiproliferative assay | 72 hrs | Antiproliferative activity against mouse B16-BL6 cells assessed as cell viability after 72 hrs by MTT assay, IC50 = 0.6 μM. | 11575942 | ||
| HT1080 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HT1080 cells assessed as cell viability after 72 hrs by MTT assay, IC50 = 1.5 μM. | 11575942 | ||
| L1210 | Growth inhibition assay | 48 hrs | Growth inhibition in L1210 mouse leukemia cells after 48 hr treatment, IC50 = 0.2 μM. | 11728193 | ||
| L1210 | Growth inhibition assay | 24 hrs | Growth inhibition in L1210 mouse leukemia cells after 24 h treatment, IC50 = 0.36 μM. | 11728193 | ||
| L1210 | Growth inhibition assay | 8 hrs | Growth inhibition in L1210 mouse leukemia cells after 8 h treatment, IC50 = 1.01 μM. | 11728193 | ||
| L1210 | Growth inhibition assay | 2 hrs | Growth inhibition in L1210 mouse leukemia cells after 2 hr treatment, IC50 = 2.5 μM. | 11728193 | ||
| HL60 | Function assay | In vitro inhibitory concentration against proliferation of HL60 cells, IC50 = 1.5 μM. | 12877593 | |||
| HT1080 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HT1080 cells after 72 hrs by MTT assay, IC50 = 3.7 μM. | 14640513 | ||
| HCT116 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human HCT116 cells after 96 hrs by MTT assay, IC50 = 4.93 μM. | 15387639 | ||
| DLD1 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human DLD1 cells after 96 hrs by MTT assay, IC50 = 7.38 μM. | 15387639 | ||
| HeLaS3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HeLaS3 cells after 72 hrs by WST1 assay, IC50 = 5.4 μM. | 15387656 | ||
| HeLaS3 | Cytotoxicity assay | Cytotoxicity against human HeLaS3 cells by WST1 assay, IC50 = 5.4 μM. | 15568771 | |||
| MCF-7 | Antiproliferative assay | Antiproliferative activity in MCF-7 human breast cancer cells, IC50 = 4.8 μM. | 15658875 | |||
| MDA-MB 231 | Antiproliferative assay | Antiproliferative activity in MDA-MB 231 human breast cancer cells, IC50 = 9.6 μM. | 15658875 | |||
| A549 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human A549 cells after 96 hrs by MTT assay, IC50 = 1.4 μM. | 16499317 | ||
| HCT8 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human HCT8 cells after 96 hrs by MTT assay, IC50 = 4.2 μM. | 16499317 | ||
| BEL-7402 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human BEL-7402 cells after 96 hrs by MTT assay, IC50 = 4.2 μM. | 16499317 | ||
| A2780 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human A2780 cells after 96 hrs by MTT assay, IC50 = 5 μM. | 16499317 | ||
| BGC823 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human BGC823 cells after 96 hrs by MTT assay, IC50 = 5.4 μM. | 16499317 | ||
| HCT116 | Cytotoxicity assay | Cytotoxicity against human HCT116 cells, IC50 = 2.04 μM. | 16996657 | |||
| COLO205 | Cytotoxicity assay | Cytotoxicity against human COLO205 cells, IC50 = 3.39 μM. | 16996657 | |||
| RPMI8226 | Cytotoxicity assay | Cytotoxicity against human RPMI8226 cells, IC50 = 3.55 μM. | 16996657 | |||
| HCC2998 | Cytotoxicity assay | Cytotoxicity against human HCC2998 cells, IC50 = 5.75 μM. | 16996657 | |||
| HCT15 | Cytotoxicity assay | Cytotoxicity against human HCT15 cells, IC50 = 6.61 μM. | 16996657 | |||
| KM12 | Cytotoxicity assay | Cytotoxicity against human KM12 cells, IC50 = 7.94 μM. | 16996657 | |||
| SW620 | Cytotoxicity assay | Cytotoxicity against human SW620 cells, IC50 = 18.6 μM. | 16996657 | |||
| KB | Cytotoxicity assay | 48 hrs | Cytotoxicity against human KB cells after 48 hrs, IC50 = 13.4 μM. | 17000031 | ||
| HT29 | Antiproliferative assay | Antiproliferative activity against HT29 cells, IC50 = 7.3 μM. | 17110105 | |||
| Vero | Cytotoxicity assay | Cytotoxicity against Vero cells, IC50 = 0.2 μM. | 17234303 | |||
| SW1116 | Cytotoxicity assay | Cytotoxicity against SW1116 cells by MTT colometric method, IC50 = 37 μM. | 17253861 | |||
| HL60 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HL60 cells after 48 hrs by MTT assay, IC50 = 0.16 μM. | 17291040 | ||
| Bel7402 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human Bel7402 cells after 48 hrs by MTT assay, IC50 = 23.21 μM. | 17291040 | ||
| BGC823 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human BGC823 cells after 48 hrs by MTT assay, IC50 = 31.16 μM. | 17291040 | ||
| HT29 | Cytotoxicity assay | Cytotoxicity against human HT29 cells by MTT microassay, IC50 = 0.6 μM. | 17320246 | |||
| Hep 3B | Cytotoxicity assay | Cytotoxicity against human Hep 3B cells by MTT microassay, IC50 = 0.6 μM. | 17320246 | |||
| MCF7 | Cytotoxicity assay | Cytotoxicity against human MCF7 cells by MTT microassay, IC50 = 1.5 μM. | 17320246 | |||
| A549 | Cytotoxicity assay | Cytotoxicity against human A549 cells by MTT microassay, IC50 = 3.1 μM. | 17320246 | |||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs, IC50 = 4.8 μM. | 17336074 | ||
| MDA-MB-231 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MDA-MB-231 cells after 72 hrs, IC50 = 9.6 μM. | 17336074 | ||
| Caco-2 | Growth inhibition assay | 10 days | Growth inhibition of human Caco-2 cells after 10 days, IC50 = 1.5 μM. | 17337193 | ||
| L1210/0 | Cytotoxicity assay | 48 hrs | Cytotoxicity against mouse L1210/0 cells after 48 hrs, IC50 = 0.31 μM. | 17341060 | ||
| HCT116 | Antitumor assay | Antitumor activity against human HCT116 cells, GI50 = 0.39811 μM. | 17446077 | |||
| NCI-H460 | Antitumor assay | Antitumor activity against human NCI-H460 cells, GI50 = 1 μM. | 17446077 | |||
| SF539 | Antitumor assay | Antitumor activity against human SF539 cells, GI50 = 1.25893 μM. | 17446077 | |||
| MCF7 | Antitumor assay | Antitumor activity against human MCF7 cells, GI50 = 1.58489 μM. | 17446077 | |||
| HCC2998 | Antitumor assay | Antitumor activity against human HCC2998 cells, GI50 = 1.58489 μM. | 17446077 | |||
| A549 | Antitumor assay | Antitumor activity against human A549 cells, GI50 = 1.99526 μM. | 17446077 | |||
| CAKI-1 | Antitumor assay | Antitumor activity against human CAKI-1 cells, GI50 = 3.98107 μM. | 17446077 | |||
| SR | Antitumor assay | Antitumor activity against human SR cells, GI50 = 3.98107 μM. | 17446077 | |||
| UO31 | Antitumor assay | Antitumor activity against human UO31 cells, GI50 = 5.01187 μM. | 17446077 | |||
| RPMI8226 | Antitumor assay | Antitumor activity against human RPMI8226 cells, GI50 = 5.01187 μM. | 17446077 | |||
| HCT15 | Antitumor assay | Antitumor activity against human HCT15 cells, GI50 = 6.30957 μM. | 17446077 | |||
| HT29 | Antitumor assay | Antitumor activity against human HT29 cells, GI50 = 6.30957 μM. | 17446077 | |||
| LOX IMVI | Antitumor assay | Antitumor activity against human LOX IMVI cells, GI50 = 6.30957 μM. | 17446077 | |||
| COLO205 | Antitumor assay | Antitumor activity against human COLO205 cells, GI50 = 6.30957 μM. | 17446077 | |||
| KM12 | Antitumor assay | Antitumor activity against human KM12 cells, GI50 = 10 μM. | 17446077 | |||
| ACHN | Antitumor assay | Antitumor activity against human ACHN cells, GI50 = 10 μM. | 17446077 | |||
| DU145 | Antitumor assay | Antitumor activity against human DU145 cells, GI50 = 10 μM. | 17446077 | |||
| A498 | Antitumor assay | Antitumor activity against human A498 cells, GI50 = 10 μM. | 17446077 | |||
| MDA-MB-435 | Antitumor assay | Antitumor activity against human MDA-MB-435 cells, GI50 = 10 μM. | 17446077 | |||
| MOLT4 | Antitumor assay | Antitumor activity against human MOLT4 cells, GI50 = 12.5892 μM. | 17446077 | |||
| NCI-H23 | Antitumor assay | Antitumor activity against human NCI-H23 cells, GI50 = 12.5892 μM. | 17446077 | |||
| IGROV1 | Antitumor assay | Antitumor activity against human IGROV1 cells, GI50 = 12.5892 μM. | 17446077 | |||
| UACC62 | Antitumor assay | Antitumor activity against human UACC62 cells, GI50 = 12.5892 μM. | 17446077 | |||
| SK-MEL-5 | Antitumor assay | Antitumor activity against human SK-MEL-5 cells, GI50 = 12.5892 μM. | 17446077 | |||
| 786-0 | Antitumor assay | Antitumor activity against human 786-0 cells, GI50 = 12.5892 μM. | 17446077 | |||
| K562 | Antitumor assay | Antitumor activity against human K562 cells, GI50 = 19.9526 μM. | 17446077 | |||
| HOP62 | Antitumor assay | Antitumor activity against human HOP62 cells, GI50 = 19.9526 μM. | 17446077 | |||
| MALME-3M | Antitumor assay | Antitumor activity against human MALME-3M cells, GI50 = 19.9526 μM. | 17446077 | |||
| OVCAR8 | Antitumor assay | Antitumor activity against human OVCAR8 cells, GI50 = 19.9526 μM. | 17446077 | |||
| HL60 (TB) | Antitumor assay | Antitumor activity against human HL60 (TB) cells, GI50 = 19.9526 μM. | 17446077 | |||
| NCI-H322 | Antitumor assay | Antitumor activity against human NCI-H322 cells, GI50 = 19.9526 μM. | 17446077 | |||
| SN12C | Antitumor assay | Antitumor activity against human SN12C cells, GI50 = 25.1189 μM. | 17446077 | |||
| OVCAR-3 | Antitumor assay | Antitumor activity against human OVCAR-3 cells, GI50 = 25.1189 μM. | 17446077 | |||
| SW620 | Antitumor assay | Antitumor activity against human SW620 cells, GI50 = 25.1189 μM. | 17446077 | |||
| CCRF-CEM | Antitumor assay | Antitumor activity against human CCRF-CEM cells, GI50 = 31.6228 μM. | 17446077 | |||
| U251 | Antitumor assay | Antitumor activity against human U251 cells, GI50 = 39.8107 μM. | 17446077 | |||
| NCI-H522 | Antitumor assay | Antitumor activity against human NCI-H522 cells, GI50 = 39.8107 μM. | 17446077 | |||
| NCI/ADR-RES | Antitumor assay | Antitumor activity against human NCI/ADR-RES cells, GI50 = 39.8107 μM. | 17446077 | |||
| SW480 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SW480 cells after 72 hrs by MTT method, IC50 = 8.1 μM. | 17524655 | ||
| MDA-MB-435S | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MDA-MB-435S cells after 72 hrs by MTT method, IC50 = 14.5 μM. | 17524655 | ||
| HCCLM7 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCCLM7 cells after 72 hrs by MTT method, IC50 = 18.6 μM. | 17524655 | ||
| Caco-2 | Growth inhibition assay | Growth inhibition of human Caco-2 cells, IC50 = 1.5 μM. | 17548129 | |||
| MCF7 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MCF7 cells after 72 hrs, IC50 = 0.3 μM. | 17548198 | ||
| HCT116 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT116 cells after 72 hrs, IC50 = 2.8 μM. | 17548198 | ||
| SW480 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SW480 cells after 72 hrs, IC50 = 3.5 μM. | 17548198 | ||
| SW48 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SW48 cells after 72 hrs, IC50 = 5.3 μM. | 17548198 | ||
| DLD1 | Growth inhibition assay | Growth inhibition of human DLD1 cells, IC50 = 4.5 μM. | 17553685 | |||
| Caco-2 | Growth inhibition assay | 10 days | Growth inhibition of human Caco-2 cells after 10 days, IC50 = 1.5 μM. | 17555969 | ||
| colon cancer cell | Cytotoxicity assay | Cytotoxicity against human colon cancer cells, GI50 = 5.75 μM. | 17562365 | |||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against A549 cells after 48 hrs by MTT assay, IC50 = 9.2 μM. | 17604394 | ||
| L1210 | Cytostatic activity assay | Cytostatic activity against mouse L1210 cells, IC50 = 0.28 μM. | 17931862 | |||
| CEM | Cytostatic activity assay | Cytostatic activity against human CEM cells, IC50 = 9 μM. | 17931862 | |||
| Molt4/C8 | Cytostatic activity assay | Cytostatic activity against human Molt4/C8 cells, IC50 = 23 μM. | 17931862 | |||
| Caco-2 | Growth inhibition assay | 10 days | Growth inhibition of human Caco-2 cells after 10 days, IC50 = 1.5 μM. | 18037195 | ||
| MCF7 | Antiproliferative assay | 3 days | Antiproliferative activity against human MCF7 cells after 3 days by SRB assay, IC50 = 4.32 μM. | 18069093 | ||
| MCF7 | Antiproliferative assay | Antiproliferative activity against human MCF7 cells, IC50 = 4.32 μM. | 18194866 | |||
| embryonic cell | Function assay | Induction of cleavage arrest in embryonic cells of stage 8 Xenopus laevis blastulae, IC50 = 26.8 μM. | 18271558 | |||
| HepG2 | Cytotoxicity assay | Cytotoxicity against human HepG2 cells, IC50 = 18.7 μM. | 18302336 | |||
| L1210 | Antiproliferative assay | 2 days | Antiproliferative activity against mouse L1210 cells after 2 days by MTT assay, IC50 = 0.69 μM. | 18424155 | ||
| MCF7 | Antiproliferative assay | 3 days | Antiproliferative activity against human MCF7 cells after 3 days by MTT assay, IC50 = 4.5 μM. | 18424155 | ||
| MiaPaCa2 | Antiproliferative assay | 3 days | Antiproliferative activity against human MiaPaCa2 cells after 3 days by MTT assay, IC50 = 6.5 μM. | 18424155 | ||
| SW620 | Antiproliferative assay | 3 days | Antiproliferative activity against human SW620 cells after 3 days by MTT assay, IC50 = 8.7 μM. | 18424155 | ||
| HepG2 | Antiproliferative assay | 3 days | Antiproliferative activity against human HepG2 cells after 3 days by MTT assay, IC50 = 9.2 μM. | 18424155 | ||
| CEM | Antiproliferative assay | 3 days | Antiproliferative activity against human CEM cells after 3 days by MTT assay, IC50 = 9.23 μM. | 18424155 | ||
| WI38 | Cytotoxicity assay | 3 days | Cytotoxicity against human WI38 cells after 3 days by MTT assay, IC50 = 10 μM. | 18424155 | ||
| HeLa | Antiproliferative assay | 3 days | Antiproliferative activity against human HeLa cells after 3 days by MTT assay, IC50 = 16 μM. | 18424155 | ||
| Molt4/C8 | Antiproliferative assay | 3 days | Antiproliferative activity against human Molt4/C8 cells after 3 days by MTT assay, IC50 = 20 μM. | 18424155 | ||
| colon 26-L5 | Cytotoxicity assay | 72 hrs | Cytotoxicity against mouse colon 26-L5 cells after 72 hrs by MTT assay, IC50 = 0.46 μM. | 18440233 | ||
| HeLa | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HeLa cells after 72 hrs by MTT assay, IC50 = 0.77 μM. | 18440233 | ||
| LLC | Cytotoxicity assay | 72 hrs | Cytotoxicity against mouse LLC cells after 72 hrs by MTT assay, IC50 = 2.59 μM. | 18440233 | ||
| B16-BL6 | Cytotoxicity assay | 72 hrs | Cytotoxicity against mouse B16-BL6 cells after 72 hrs by MTT assay, IC50 = 5.78 μM. | 18440233 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells after 72 hrs by MTT assay, IC50 = 9.19 μM. | 18440233 | ||
| HT1080 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HT1080 cells after 72 hrs by MTT assay, IC50 = 9.45 μM. | 18440233 | ||
| HCT116 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT116 cells expressing p53 gene after 72 hrs by proliferative assay, IC50 = 6.36 μM. | 18469809 | ||
| A2780 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A2780 cells expressing p53 gene after 72 hrs by proliferative assay, IC50 = 7.21 μM. | 18469809 | ||
| COLO205 | Antiproliferative assay | 72 hrs | Antiproliferative activity against p53 deficient human COLO205 cells after 72 hrs, IC50 = 11.63 μM. | 18469809 | ||
| U2OS | Antiproliferative assay | 72 hrs | Antiproliferative activity against human U2OS cells expressing p53 gene after 72 hrs by proliferative assay, IC50 = 13.18 μM. | 18469809 | ||
| PC3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human PC3 cells expressing p53 gene after 72 hrs by proliferative assay, IC50 = 13.77 μM. | 18469809 | ||
| HCT15 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT15 cells expressing p53 gene after 72 hrs by proliferative assay, IC50 = 18.24 μM. | 18469809 | ||
| SW480 | Antiproliferative assay | 72 hrs | Antiproliferative activity against p53 deficient human SW480 cells after 72 hrs by proliferative assay, IC50 = 26.26 μM. | 18469809 | ||
| MCF7 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MCF7 cells expressing p53 gene after 72 hrs by proliferative assay, IC50 = 26.67 μM. | 18469809 | ||
| HCT116/E6 | Antiproliferative assay | 72 hrs | Antiproliferative activity against p53 deficient human HCT116/E6 cells after 72 hrs by proliferative assay, IC50 = 28.91 μM. | 18469809 | ||
| A2780/E6 | Antiproliferative assay | 72 hrs | Antiproliferative activity against p53 deficient human A2780/E6 cells after 72 hrs by proliferative assay, IC50 = 38.67 μM. | 18469809 | ||
| HL60 | Antiproliferative assay | 3 days | Antiproliferative activity against human HL60 cells after 3 days by trypan blue staining, GI50 = 3.8 μM. | 18491866 | ||
| HT29 | Cytotoxicity assay | Cytotoxicity against human HT29 cells by MTT assay, IC50 = 0.6 μM. | 18606546 | |||
| Hep3B | Cytotoxicity assay | Cytotoxicity against human Hep3B cells by MTT assay, IC50 = 0.6 μM. | 18606546 | |||
| MCF7 | Cytotoxicity assay | Cytotoxicity against human MCF7 cells by MTT assay, IC50 = 1.5 μM. | 18606546 | |||
| A549 | Cytotoxicity assay | Cytotoxicity against human A549 cells by MTT assay, IC50 = 3.1 μM. | 18606546 | |||
| HaCaT | Growth inhibition assay | 72 hrs | Growth inhibition of human HaCaT cells after 72 hrs by MTT assay, IC50 = 0.4 μM. | 18640746 | ||
| H460 | Growth inhibition assay | 72 hrs | Growth inhibition of human H460 cells after 72 hrs by MTT assay, IC50 = 2 μM. | 18640746 | ||
| HeLa | Growth inhibition assay | 72 hrs | Growth inhibition of human HeLa cells after 72 hrs by MTT assay, IC50 = 4 μM. | 18640746 | ||
| SW620 | Growth inhibition assay | 72 hrs | Growth inhibition of human SW620 cells after 72 hrs by MTT assay, IC50 = 9 μM. | 18640746 | ||
| MIAPaCa2 | Growth inhibition assay | 72 hrs | Growth inhibition of human MIAPaCa2 cells after 72 hrs by MTT assay, IC50 = 10 μM. | 18640746 | ||
| MCF7 | Growth inhibition assay | 72 hrs | Growth inhibition of human MCF7 cells after 72 hrs by MTT assay, IC50 = 15 μM. | 18640746 | ||
| B16 | Cytotoxicity assay | Cytotoxicity against mouse B16 cells by MTT assay, IC50 = 8.73 μM. | 18644718 | |||
| A549 | Cytotoxicity assay | Cytotoxicity against human A549 cells by MTT assay, IC50 = 10.85 μM. | 18644718 | |||
| SW480 | Cytotoxicity assay | Cytotoxicity against human SW480 cells by MTT assay, IC50 = 13.16 μM. | 18644718 | |||
| BEL-7402 | Cytotoxicity assay | Cytotoxicity against human BEL-7402 cells by MTT assay, IC50 = 14.41 μM. | 18644718 | |||
| BGC-7901 | Cytotoxicity assay | Cytotoxicity against human BGC-7901 cells by MTT assay, IC50 = 16.35 μM. | 18644718 | |||
| HL60 | Cytotoxicity assay | Cytotoxicity against human HL60 cells by MTT assay, IC50 = 21.38 μM. | 18644718 | |||
| 3T3 | Cytotoxicity assay | 48 hrs | Cytotoxicity against BALB mouse 3T3 cells after 48 hrs, GI50 = 1.54 μM. | 18798609 | ||
| H460 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human H460 cells after 48 hrs, GI50 = 4.1 μM. | 18798609 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MCF7 cells after 48 hrs, GI50 = 6.5 μM. | 18798609 | ||
| HT29 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HT29 cells after 48 hrs, GI50 = 8.9 μM. | 18798609 | ||
| PC3 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human PC3 cells after 48 hrs, GI50 = 14.6 μM. | 18798609 | ||
| DU145 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human DU145 cells after 48 hrs, GI50 = 15.4 μM. | 18798609 | ||
| K562 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human K562 cells after 48 hrs, GI50 = 16.7 μM. | 18798609 | ||
| M14 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human M14 cells after 48 hrs, GI50 = 22.4 μM. | 18798609 | ||
| colon cancer cell | Growth inhibition assay | 48 hrs | Growth inhibition of human Colon cancer cells after 48 hrs, GI50 = 8.37 μM. | 18950904 | ||
| leukemia cell | Growth inhibition assay | 48 hrs | Growth inhibition of human leukemia cells after 48 hrs, GI50 = 15.09 μM. | 18950904 | ||
| prostate cancer cell | Growth inhibition assay | 48 hrs | Growth inhibition of human prostate cancer cells after 48 hrs, GI50 = 22.7 μM. | 18950904 | ||
| renal cancer cell | Growth inhibition assay | 48 hrs | Growth inhibition of human renal cancer cells after 48 hrs, GI50 = 45.6 μM. | 18950904 | ||
| KB | Cytotoxicity assay | Cytotoxicity against human KB cells by MTT assay, IC50 = 0.00446 μM. | 19345581 | |||
| NCI60 | Growth inhibition assay | Growth inhibition of human NCI60 cells, GI50 = 17.7 μM. | 19398334 | |||
| A549 | Cytotoxicity assay | Cytotoxicity against human A549 cells by SRB method, IC50 = 20.8 μM. | 19422203 | |||
| PC3 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human PC3 cells after 48 hrs by MTT assay, IC50 = 0.016 μM. | 19423198 | ||
| A431 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human A431 cells after 48 hrs by MTT assay, IC50 = 0.018 μM. | 19423198 | ||
| MCF7 | Antiproliferative assay | 24 hrs | Antiproliferative activity against human MCF7 cells after 24 hrs by MTT assay, IC50 = 4.3 μM. | 19560842 | ||
| Bel7402 | Antiproliferative assay | 24 hrs | Antiproliferative activity against human Bel7402 cells after 24 hrs by MTT assay, IC50 = 4.7 μM. | 19560842 | ||
| HeLa | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HeLa cells after 48 hrs by MTT assay, GI50 = 10 μM. | 19572613 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MCF7 cells after 48 hrs by MTT assay, GI50 = 15 μM. | 19572613 | ||
| HeLa | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HeLa cells after 48 hrs by MTT assay, IC50 = 10 μM. | 19618920 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MCF7 cells after 48 hrs by MTT assay, IC50 = 15 μM. | 19618920 | ||
| SW1573 | Growth inhibition assay | 48 hrs | Growth inhibition of human SW1573 cells after 48 hrs by sulforhodamine B assay, GI50 = 4.1 μM. | 19664930 | ||
| A2780 | Growth inhibition assay | 48 hrs | Growth inhibition of human A2780 cells after 48 hrs by sulforhodamine B assay, GI50 = 4.4 μM. | 19664930 | ||
| HBL100 | Growth inhibition assay | 48 hrs | Growth inhibition of human HBL100 cells after 48 hrs by sulforhodamine B assay, GI50 = 5.5 μM. | 19664930 | ||
| HeLa | Growth inhibition assay | 48 hrs | Growth inhibition of human HeLa cells after 48 hrs by sulforhodamine B assay, GI50 = 19 μM. | 19664930 | ||
| T47D | Growth inhibition assay | 48 hrs | Growth inhibition of human T47D cells after 48 hrs by sulforhodamine B assay, GI50 = 29 μM. | 19664930 | ||
| Colon 26-L5 | Cytotoxicity assay | 72 hrs | Cytotoxicity against mouse Colon 26-L5 cells after 72 hrs by MTT assay, IC50 = 0.41 μM. | 19689125 | ||
| HeLa | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HeLa cells after 72 hrs by MTT assay, IC50 = 0.44 μM. | 19689125 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells after 72 hrs by MTT assay, IC50 = 5.91 μM. | 19689125 | ||
| B16-BL6 | Cytotoxicity assay | 72 hrs | Cytotoxicity against mouse B16-BL6 cells after 72 hrs by MTT assay, IC50 = 7.39 μM. | 19689125 | ||
| HT1080 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HT1080 cells after 72 hrs by MTT assay, IC50 = 8.58 μM. | 19689125 | ||
| OVCAR-3 | Anticancer assay | 48 hrs | Anticancer activity against human OVCAR-3 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.01585 μM. | 19740574 | ||
| SR | Anticancer assay | 48 hrs | Anticancer activity against human SR cells after 48 hrs by sulforhodamine B assay, GI50 = 0.02399 μM. | 19740574 | ||
| RPMI8266 | Anticancer assay | 48 hrs | Anticancer activity against human RPMI8266 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.04467 μM. | 19740574 | ||
| MALME-3M | Anticancer assay | 48 hrs | Anticancer activity against human MALME-3M cells after 48 hrs by sulforhodamine B assay, GI50 = 0.05129 μM. | 19740574 | ||
| HCC2998 | Anticancer assay | 48 hrs | Anticancer activity against human HCC2998 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.05248 μM. | 19740574 | ||
| NCI-H460 | Anticancer assay | 48 hrs | Anticancer activity against human NCI-H460 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.05623 μM. | 19740574 | ||
| SF539 | Anticancer assay | 48 hrs | Anticancer activity against human SF539 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.0631 μM. | 19740574 | ||
| Caki1 | Anticancer assay | 48 hrs | Anticancer activity against human Caki1 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.07244 μM. | 19740574 | ||
| MDA-MB-435 | Anticancer assay | 48 hrs | Anticancer activity against human MDA-MB-435 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.07244 μM. | 19740574 | ||
| MCF7 | Anticancer assay | 48 hrs | Anticancer activity against human MCF7 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.07943 μM. | 19740574 | ||
| HCT15 | Anticancer assay | 48 hrs | Anticancer activity against human HCT15 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.10965 μM. | 19740574 | ||
| COLO205 | Anticancer assay | 48 hrs | Anticancer activity against human COLO205 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.15849 μM. | 19740574 | ||
| NCI-H322M | Anticancer assay | 48 hrs | Anticancer activity against human NCI-H322M cells after 48 hrs by sulforhodamine B assay, GI50 = 0.17783 μM. | 19740574 | ||
| HT-29 | Anticancer assay | 48 hrs | Anticancer activity against human HT-29 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.17783 μM. | 19740574 | ||
| A549 | Anticancer assay | 48 hrs | Anticancer activity against human A549 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.19055 μM. | 19740574 | ||
| KM12 | Anticancer assay | 48 hrs | Anticancer activity against human KM12 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.2138 μM. | 19740574 | ||
| HCT116 | Anticancer assay | 48 hrs | Anticancer activity against human HCT116 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.22909 μM. | 19740574 | ||
| SF295 | Anticancer assay | 48 hrs | Anticancer activity against human SF295 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.22909 μM. | 19740574 | ||
| LOXIMVI | Anticancer assay | 48 hrs | Anticancer activity against human LOXIMVI cells after 48 hrs by sulforhodamine B assay, GI50 = 0.24547 μM. | 19740574 | ||
| ACHN | Anticancer assay | 48 hrs | Anticancer activity against human ACHN cells after 48 hrs by sulforhodamine B assay, GI50 = 0.29512 μM. | 19740574 | ||
| NCI/ADR-RES | Anticancer assay | 48 hrs | Anticancer activity against human NCI/ADR-RES cells after 48 hrs by sulforhodamine B assay, GI50 = 0.31623 μM. | 19740574 | ||
| NCI-H23 | Anticancer assay | 48 hrs | Anticancer activity against human NCI-H23 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.33113 μM. | 19740574 | ||
| MOLT4 | Anticancer assay | 48 hrs | Anticancer activity against human MOLT4 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.35481 μM. | 19740574 | ||
| DU145 | Anticancer assay | 48 hrs | Anticancer activity against human DU145 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.36308 μM. | 19740574 | ||
| HOP62 | Anticancer assay | 48 hrs | Anticancer activity against human HOP62 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.39811 μM. | 19740574 | ||
| A498 | Anticancer assay | 48 hrs | Anticancer activity against human A498 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.39811 μM. | 19740574 | ||
| SK-MEL-5 | Anticancer assay | 48 hrs | Anticancer activity against human SK-MEL-5 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.46774 μM. | 19740574 | ||
| SN12C | Anticancer assay | 48 hrs | Anticancer activity against human SN12C cells after 48 hrs by sulforhodamine B assay, GI50 = 0.50119 μM. | 19740574 | ||
| UACC62 | Anticancer assay | 48 hrs | Anticancer activity against human UACC62 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.52481 μM. | 19740574 | ||
| 786-0 | Anticancer assay | 48 hrs | Anticancer activity against human 786-0 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.72444 μM. | 19740574 | ||
| U251 | Anticancer assay | 48 hrs | Anticancer activity against human U251 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.91201 μM. | 19740574 | ||
| SW620 | Anticancer assay | 48 hrs | Anticancer activity against human SW620 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.93325 μM. | 19740574 | ||
| M14 | Anticancer assay | 48 hrs | Anticancer activity against human M14 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.97724 μM. | 19740574 | ||
| SK-MEL-28 | Anticancer assay | 48 hrs | Anticancer activity against human SK-MEL-28 cells after 48 hrs by sulforhodamine B assay, GI50 = 1.02329 μM. | 19740574 | ||
| TK10 | Anticancer assay | 48 hrs | Anticancer activity against human TK10 cells after 48 hrs by sulforhodamine B assay, GI50 = 1.12202 μM. | 19740574 | ||
| IGROV1 | Anticancer assay | 48 hrs | Anticancer activity against human IGROV1 cells after 48 hrs by sulforhodamine B assay, GI50 = 1.23027 μM. | 19740574 | ||
| UO31 | Anticancer assay | 48 hrs | Anticancer activity against human UO31 cells after 48 hrs by sulforhodamine B assay, GI50 = 1.41254 μM. | 19740574 | ||
| SF268 | Anticancer assay | 48 hrs | Anticancer activity against human SF268 cells after 48 hrs by sulforhodamine B assay, GI50 = 1.58489 μM. | 19740574 | ||
| OVCAR8 | Anticancer assay | 48 hrs | Anticancer activity against human OVCAR8 cells after 48 hrs by sulforhodamine B assay, GI50 = 1.7378 μM. | 19740574 | ||
| HL-60(TB) | Anticancer assay | 48 hrs | Anticancer activity against human HL-60(TB) cells after 48 hrs by sulforhodamine B assay, GI50 = 2.29087 μM. | 19740574 | ||
| PC3 | Anticancer assay | 48 hrs | Anticancer activity against human PC3 cells after 48 hrs by sulforhodamine B assay, GI50 = 2.34423 μM. | 19740574 | ||
| RXF393 | Anticancer assay | 48 hrs | Anticancer activity against human RXF393 cells after 48 hrs by sulforhodamine B assay, GI50 = 2.63027 μM. | 19740574 | ||
| K562 | Anticancer assay | 48 hrs | Anticancer activity against human K562 cells after 48 hrs by sulforhodamine B assay, GI50 = 3.54813 μM. | 19740574 | ||
| UACC257 | Anticancer assay | 48 hrs | Anticancer activity against human UACC257 cells after 48 hrs by sulforhodamine B assay, GI50 = 3.54813 μM. | 19740574 | ||
| SNB19 | Anticancer assay | 48 hrs | Anticancer activity against human SNB19 cells after 48 hrs by sulforhodamine B assay, GI50 = 3.80189 μM. | 19740574 | ||
| OVCAR4 | Anticancer assay | 48 hrs | Anticancer activity against human OVCAR4 cells after 48 hrs by sulforhodamine B assay, GI50 = 4.46684 μM. | 19740574 | ||
| MDA-MB-231 | Anticancer assay | 48 hrs | Anticancer activity against human MDA-MB-231 cells after 48 hrs by sulforhodamine B assay, GI50 = 6.60693 μM. | 19740574 | ||
| NCI-H522 | Anticancer assay | 48 hrs | Anticancer activity against human NCI-H522 cells after 48 hrs by sulforhodamine B assay, GI50 = 7.24436 μM. | 19740574 | ||
| T47D | Anticancer assay | 48 hrs | Anticancer activity against human T47D cells after 48 hrs by sulforhodamine B assay, GI50 = 8.12831 μM. | 19740574 | ||
| CCRF-CEM | Anticancer assay | 48 hrs | Anticancer activity against human CCRF-CEM cells after 48 hrs by sulforhodamine B assay, GI50 = 9.77237 μM. | 19740574 | ||
| Hs 578T | Anticancer assay | 48 hrs | Anticancer activity against human Hs 578T cells after 48 hrs by sulforhodamine B assay, GI50 = 9.77237 μM. | 19740574 | ||
| BT549 | Anticancer assay | 48 hrs | Anticancer activity against human BT549 cells after 48 hrs by sulforhodamine B assay, GI50 = 10.7152 μM. | 19740574 | ||
| OVCAR5 | Anticancer assay | 48 hrs | Anticancer activity against human OVCAR5 cells after 48 hrs by sulforhodamine B assay, GI50 = 10.9648 μM. | 19740574 | ||
| SKOV3 | Anticancer assay | 48 hrs | Anticancer activity against human SKOV3 cells after 48 hrs by sulforhodamine B assay, GI50 = 21.8776 μM. | 19740574 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by MTT assay, IC50 = 0.3 μM. | 19743863 | ||
| T47D | Antiproliferative assay | 72 hrs | Antiproliferative activity against human T47D cells after 72 hrs by MTT assay, IC50 = 0.6 μM. | 19743863 | ||
| MES-SA | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MES-SA cells after 72 hrs by MTT assay, IC50 = 1.1 μM. | 19743863 | ||
| SW480 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SW480 cells after 72 hrs by MTT assay, IC50 = 2.1 μM. | 19743863 | ||
| HCT116 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT116 cells after 72 hrs by MTT assay, IC50 = 2.6 μM. | 19743863 | ||
| UACC-812 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human UACC-812 cells after 72 hrs by MTT assay, IC50 = 3.1 μM. | 19743863 | ||
| RL | Antiproliferative assay | 72 hrs | Antiproliferative activity against human RL cells after 72 hrs by MTT assay, IC50 = 3.2 μM. | 19743863 | ||
| SW48 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SW48 cells after 72 hrs by MTT assay, IC50 = 4.4 μM. | 19743863 | ||
| HeLa | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HeLa cells after 48 hrs by MTT assay, IC50 = 10 μM. | 19795843 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MCF7 cells after 48 hrs by MTT assay, IC50 = 15 μM. | 19795843 | ||
| KB | Cytotoxicity assay | 72 hrs | Cytotoxicity against human KB cells after 72 hrs MTT assay, IC50 = 12.5 μM. | 19846302 | ||
| HT1080 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HT1080 cells after 72 hrs by MTT assay, IC50 = 5.2 μM. | 19919052 | ||
| HeLa | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HeLa cells after 48 hrs by MTT assay, IC50 = 10 μM. | 19919065 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MCF7 cells after 48 hrs by MTT assay, IC50 = 15 μM. | 19919065 | ||
| COLO205 | Growth inhibition assay | 48 hrs | Growth inhibition of human COLO205 cells after 48 hrs by sulforhodamine B assay, IC50 = 21 μM. | 19939522 | ||
| HCT116 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human HCT116 cells after 24 hrs by trypan blue exclusion assay, IC50 = 3.03 μM. | 20163895 | ||
| MOLT4 | Antitumor assay | 24 hrs | Antitumor activity against human MOLT4 cells after 24 hrs by MTT assay, IC50 = 0.03 μM. | 20211564 | ||
| H460 | Antitumor assay | 24 hrs | Antitumor activity against human H460 cells after 24 hrs by MTT assay, IC50 = 2 μM. | 20211564 | ||
| HCT116 | Antitumor assay | 24 hrs | Antitumor activity against human HCT116 cells after 24 hrs by MTT assay, IC50 = 4 μM. | 20211564 | ||
| SW620 | Antitumor assay | 24 hrs | Antitumor activity against human SW620 cells after 24 hrs by MTT assay, IC50 = 9 μM. | 20211564 | ||
| MCF7 | Antitumor assay | 24 hrs | Antitumor activity against human MCF7 cells after 24 hrs by MTT assay, IC50 = 15 μM. | 20211564 | ||
| colon cancer | Antitumor assay | 48 hrs | Antitumor activity against human colon cancer cells after 48 hrs by SRB assay, GI50 = 8.4 μM. | 20350811 | ||
| leukemia | Antitumor assay | 48 hrs | Antitumor activity against human leukemia cells after 48 hrs by SRB assay, GI50 = 15.1 μM. | 20350811 | ||
| prostate cancer | Antitumor assay | 48 hrs | Antitumor activity against human prostate cancer cells after 48 hrs by SRB assay, GI50 = 22.7 μM. | 20350811 | ||
| renal cancer | Antitumor assay | 48 hrs | Antitumor activity against human renal cancer cells after 48 hrs by SRB assay, GI50 = 45.6 μM. | 20350811 | ||
| KB | Cytotoxicity assay | 3 days | Cytotoxicity against human KB cells after 3 days by MTT assay, IC50 = 4.46 μM. | 20356655 | ||
| NCI-H460 | Cytotoxicity assay | Cytotoxicity against human NCI-H460 cells, GI50 = 2.3 μM. | 20356752 | |||
| 3T3 | Cytotoxicity assay | Cytotoxicity against BALB mouse 3T3 cells, GI50 = 3 μM. | 20356752 | |||
| MCF7 | Cytotoxicity assay | Cytotoxicity against human MCF7 cells, GI50 = 4.5 μM. | 20356752 | |||
| DU145 | Cytotoxicity assay | Cytotoxicity against human DU145 cells, GI50 = 5.3 μM. | 20356752 | |||
| HT-29 | Cytotoxicity assay | Cytotoxicity against human HT-29 cells, GI50 = 14.9 μM. | 20356752 | |||
| M14 | Cytotoxicity assay | Cytotoxicity against human M14 cells, GI50 = 32.4 μM. | 20356752 | |||
| SFME | Antiproliferative assay | 24 hrs | Antiproliferative activity against mouse SFME cells after 24 hrs by MTT assay, IC50 = 3.2 μM. | 20398972 | ||
| r/m HM-SFME-1 | Antiproliferative assay | 24 hrs | Antiproliferative activity against mouse r/m HM-SFME-1 cells after 24 hrs by MTT assay, IC50 = 48.5 μM. | 20398972 | ||
| H460 | Antiproliferative assay | 24 hrs | Antiproliferative activity against human H460 cells after 24 hrs by trypan blue exclusion assay, IC50 = 2.7 μM. | 20403701 | ||
| MDA231 | Antiproliferative assay | 24 hrs | Antiproliferative activity against human MDA231 cells after 24 hrs by trypan blue exclusion assay, IC50 = 3.5 μM. | 20403701 | ||
| A549 | Antiproliferative assay | 24 hrs | Antiproliferative activity against human A549 cells after 24 hrs by trypan blue exclusion assay, IC50 = 5.4 μM. | 20403701 | ||
| MCF7 | Antiproliferative assay | 24 hrs | Antiproliferative activity against human MCF7 cells after 24 hrs by trypan blue exclusion assay, IC50 = 15.2 μM. | 20403701 | ||
| HeLa | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HeLa cells after 48 hrs by MTT assay, IC50 = 10 μM. | 20405881 | ||
| SKBR3 | Antiproliferative assay | 48 hrs | Antiproliferative activity against estrogen receptor deficient human SKBR3 cells after 48 hrs by SRB assay, GI50 = 3 μM. | 20438092 | ||
| MCF7/BUS | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MCF7/BUS cells expressing estrogen receptor after 48 hrs by SRB assay, GI50 = 6.2 μM. | 20438092 | ||
| MCF7 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MCF7 cells expressing estrogen receptor after 48 hrs by SRB assay, GI50 = 8.3 μM. | 20438092 | ||
| T47D | Antiproliferative assay | 48 hrs | Antiproliferative activity against human T47D cells expressing estrogen receptor after 48 hrs by SRB assay, GI50 = 47 μM. | 20438092 | ||
| HCT116 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT116 cells after 72 hrs by MTT assay, IC50 = 1.93 μM. | 20494492 | ||
| DU145 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human DU145 cells after 72 hrs by MTT assay, IC50 = 2.19 μM. | 20494492 | ||
| SGC7901 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human SGC7901 cells after 72 hrs by MTT assay, IC50 = 2.95 μM. | 20494492 | ||
| HeLa | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HeLa cells after 72 hrs by MTT assay, IC50 = 9.7 μM. | 20494492 | ||
| K562 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human K562 cells after 72 hrs by MTT assay, IC50 = 45.4 μM. | 20494492 | ||
| OVCAR-3 | Growth inhibition assay | 48 hrs | Growth inhibition of human OVCAR-3 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.01585 μM. | 20850204 | ||
| SR | Growth inhibition assay | 48 hrs | Growth inhibition of human SR cells after 48 hrs by sulforhodamine B assay, GI50 = 0.02399 μM. | 20850204 | ||
| RPMI8266 | Growth inhibition assay | 48 hrs | Growth inhibition of human RPMI8266 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.04467 μM. | 20850204 | ||
| MALME-3M | Growth inhibition assay | 48 hrs | Growth inhibition of human MALME-3M cells after 48 hrs by sulforhodamine B assay, GI50 = 0.05129 μM. | 20850204 | ||
| HCC2998 | Growth inhibition assay | 48 hrs | Growth inhibition of human HCC2998 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.05248 μM. | 20850204 | ||
| NCI-H460 | Growth inhibition assay | 48 hrs | Growth inhibition of human NCI-H460 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.05623 μM. | 20850204 | ||
| SF539 | Growth inhibition assay | 48 hrs | Growth inhibition of human SF539 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.0631 μM. | 20850204 | ||
| Caki1 | Growth inhibition assay | 48 hrs | Growth inhibition of human Caki1 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.07244 μM. | 20850204 | ||
| MDA-MB-435 | Growth inhibition assay | 48 hrs | Growth inhibition of human MDA-MB-435 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.07244 μM. | 20850204 | ||
| MCF7 | Growth inhibition assay | 48 hrs | Growth inhibition of human MCF7 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.07943 μM. | 20850204 | ||
| HCT15 | Growth inhibition assay | 48 hrs | Growth inhibition of human HCT15 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.10965 μM. | 20850204 | ||
| COLO205 | Growth inhibition assay | 48 hrs | Growth inhibition of human COLO205 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.15849 μM. | 20850204 | ||
| HT-29 | Growth inhibition assay | 48 hrs | Growth inhibition of human HT-29 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.17783 μM. | 20850204 | ||
| NCI-H322M | Growth inhibition assay | 48 hrs | Growth inhibition of human NCI-H322M cells after 48 hrs by sulforhodamine B assay, GI50 = 0.17783 μM. | 20850204 | ||
| A549 | Growth inhibition assay | 48 hrs | Growth inhibition of human A549 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.19055 μM. | 20850204 | ||
| KM12 | Growth inhibition assay | 48 hrs | Growth inhibition of human KM12 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.2138 μM. | 20850204 | ||
| HCT116 | Growth inhibition assay | 48 hrs | Growth inhibition of human HCT116 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.22909 μM. | 20850204 | ||
| SF295 | Growth inhibition assay | 48 hrs | Growth inhibition of human SF295 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.22909 μM. | 20850204 | ||
| LOXIMVI | Growth inhibition assay | 48 hrs | Growth inhibition of human LOXIMVI cells after 48 hrs by sulforhodamine B assay, GI50 = 0.24547 μM. | 20850204 | ||
| ACHN | Growth inhibition assay | 48 hrs | Growth inhibition of human ACHN cells after 48 hrs by sulforhodamine B assay, GI50 = 0.29512 μM. | 20850204 | ||
| NCI/ADR-RES | Growth inhibition assay | 48 hrs | Growth inhibition of human NCI/ADR-RES cells after 48 hrs by sulforhodamine B assay, GI50 = 0.31623 μM. | 20850204 | ||
| NCI-H23 | Growth inhibition assay | 48 hrs | Growth inhibition of human NCI-H23 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.33113 μM. | 20850204 | ||
| MOLT4 | Growth inhibition assay | 48 hrs | Growth inhibition of human MOLT4 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.35481 μM. | 20850204 | ||
| DU145 | Growth inhibition assay | 48 hrs | Growth inhibition of human DU145 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.36308 μM. | 20850204 | ||
| HOP62 | Growth inhibition assay | 48 hrs | Growth inhibition of human HOP62 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.39811 μM. | 20850204 | ||
| A498 | Growth inhibition assay | 48 hrs | Growth inhibition of human A498 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.39811 μM. | 20850204 | ||
| SK-MEL-5 | Growth inhibition assay | 48 hrs | Growth inhibition of human SK-MEL-5 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.46774 μM. | 20850204 | ||
| SN12C | Growth inhibition assay | 48 hrs | Growth inhibition of human SN12C cells after 48 hrs by sulforhodamine B assay, GI50 = 0.50119 μM. | 20850204 | ||
| UACC62 | Growth inhibition assay | 48 hrs | Growth inhibition of human UACC62 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.52481 μM. | 20850204 | ||
| 786-0 | Growth inhibition assay | 48 hrs | Growth inhibition of human 786-0 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.72444 μM. | 20850204 | ||
| U251 | Growth inhibition assay | 48 hrs | Growth inhibition of human U251 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.91201 μM. | 20850204 | ||
| SW620 | Growth inhibition assay | 48 hrs | Growth inhibition of human SW620 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.93325 μM. | 20850204 | ||
| M14 | Growth inhibition assay | 48 hrs | Growth inhibition of human M14 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.97724 μM. | 20850204 | ||
| SK-MEL-28 | Growth inhibition assay | 48 hrs | Growth inhibition of human SK-MEL-28 cells after 48 hrs by sulforhodamine B assay, GI50 = 1.02329 μM. | 20850204 | ||
| TK10 | Growth inhibition assay | 48 hrs | Growth inhibition of human TK10 cells after 48 hrs by sulforhodamine B assay, GI50 = 1.12202 μM. | 20850204 | ||
| IGROV1 | Growth inhibition assay | 48 hrs | Growth inhibition of human IGROV1 cells after 48 hrs by sulforhodamine B assay, GI50 = 1.23027 μM. | 20850204 | ||
| UO31 | Growth inhibition assay | 48 hrs | Growth inhibition of human UO31 cells after 48 hrs by sulforhodamine B assay, GI50 = 1.41254 μM. | 20850204 | ||
| SF268 | Growth inhibition assay | 48 hrs | Growth inhibition of human SF268 cells after 48 hrs by sulforhodamine B assay, GI50 = 1.58489 μM. | 20850204 | ||
| OVCAR8 | Growth inhibition assay | 48 hrs | Growth inhibition of human OVCAR8 cells after 48 hrs by sulforhodamine B assay, GI50 = 1.7378 μM. | 20850204 | ||
| HL-60(TB) | Growth inhibition assay | 48 hrs | Growth inhibition of human HL-60(TB) cells after 48 hrs by sulforhodamine B assay, GI50 = 2.29087 μM. | 20850204 | ||
| PC3 | Growth inhibition assay | 48 hrs | Growth inhibition of human PC3 cells after 48 hrs by sulforhodamine B assay, GI50 = 2.34423 μM. | 20850204 | ||
| RXF393 | Growth inhibition assay | 48 hrs | Growth inhibition of human RXF393 cells after 48 hrs by sulforhodamine B assay, GI50 = 2.63027 μM. | 20850204 | ||
| K562 | Growth inhibition assay | 48 hrs | Growth inhibition of human K562 cells after 48 hrs by sulforhodamine B assay, GI50 = 3.54813 μM. | 20850204 | ||
| UACC257 | Growth inhibition assay | 48 hrs | Growth inhibition of human UACC257 cells after 48 hrs by sulforhodamine B assay, GI50 = 3.54813 μM. | 20850204 | ||
| SNB19 | Growth inhibition assay | 48 hrs | Growth inhibition of human SNB19 cells after 48 hrs by sulforhodamine B assay, GI50 = 3.80189 μM. | 20850204 | ||
| OVCAR4 | Growth inhibition assay | 48 hrs | Growth inhibition of human OVCAR4 cells after 48 hrs by sulforhodamine B assay, GI50 = 4.46684 μM. | 20850204 | ||
| MDA-MB-231 | Growth inhibition assay | 48 hrs | Growth inhibition of human MDA-MB-231 cells after 48 hrs by sulforhodamine B assay, GI50 = 6.60693 μM. | 20850204 | ||
| NCI-H522 | Growth inhibition assay | 48 hrs | Growth inhibition of human NCI-H522 cells after 48 hrs by sulforhodamine B assay, GI50 = 7.24436 μM. | 20850204 | ||
| T47D | Growth inhibition assay | 48 hrs | Growth inhibition of human T47D cells after 48 hrs by sulforhodamine B assay, GI50 = 8.12831 μM. | 20850204 | ||
| CCRF-CEM | Growth inhibition assay | 48 hrs | Growth inhibition of human CCRF-CEM cells after 48 hrs by sulforhodamine B assay, GI50 = 9.77237 μM. | 20850204 | ||
| Hs 578T | Growth inhibition assay | 48 hrs | Growth inhibition of human Hs 578T cells after 48 hrs by sulforhodamine B assay, GI50 = 9.77237 μM. | 20850204 | ||
| BT549 | Growth inhibition assay | 48 hrs | Growth inhibition of human BT549 cells after 48 hrs by sulforhodamine B assay, GI50 = 10.7152 μM. | 20850204 | ||
| OVCAR5 | Growth inhibition assay | 48 hrs | Growth inhibition of human OVCAR5 cells after 48 hrs by sulforhodamine B assay, GI50 = 10.9648 μM. | 20850204 | ||
| SKOV3 | Growth inhibition assay | 48 hrs | Growth inhibition of human SKOV3 cells after 48 hrs by sulforhodamine B assay, GI50 = 21.8776 μM. | 20850204 | ||
| HCT116 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT116 cells expressing p53 after 72 hrs by celltiter-glo assay, IC50 = 12 μM. | 20873740 | ||
| A2780 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A2780 cells expressing p53 after 72 hrs by celltiter-glo assay, IC50 = 14 μM. | 20873740 | ||
| U2OS | Antiproliferative assay | 72 hrs | Antiproliferative activity against human U2OS cells expressing p53 after 72 hrs by celltiter-glo assay, IC50 = 16 μM. | 20873740 | ||
| p53 deficient PC3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human p53 deficient PC3 cells after 72 hrs by celltiter-glo assay, IC50 = 30 μM. | 20873740 | ||
| SF539 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SF539 cells expressing p53 after 72 hrs by celltiter-glo assay, IC50 = 43 μM. | 20873740 | ||
| HCT15 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT15 cells expressing p53 after 72 hrs by celltiter-glo assay, IC50 = 48 μM. | 20873740 | ||
| T3M4 | Cytotoxicity assay | Cytotoxicity against human T3M4 cells by crystal violet staining, IC50 = 0.33 μM. | 20930123 | |||
| Colo-357 | Cytotoxicity assay | Cytotoxicity against human Colo-357 cells by crystal violet staining, IC50 = 2.05 μM. | 20930123 | |||
| Patu-T | Cytotoxicity assay | Cytotoxicity against human Patu-T cells by crystal violet staining, IC50 = 2.08 μM. | 20930123 | |||
| Patu-S | Cytotoxicity assay | Cytotoxicity against human Patu-S cells by crystal violet staining, IC50 = 2.93 μM. | 20930123 | |||
| Aspc-1 | Cytotoxicity assay | Cytotoxicity against human Aspc-1 cells by crystal violet staining, IC50 = 2.97 μM. | 20930123 | |||
| PANC1 | Cytotoxicity assay | Cytotoxicity against human PANC1 cells by crystal violet staining, IC50 = 3.94 μM. | 20930123 | |||
| Patu-02 | Cytotoxicity assay | Cytotoxicity against human Patu-02 cells by crystal violet staining, IC50 = 3.95 μM. | 20930123 | |||
| DAN-G | Cytotoxicity assay | Cytotoxicity against human DAN-G cells by crystal violet staining, IC50 = 4.38 μM. | 20930123 | |||
| Patu-T | Cytotoxicity assay | Cytotoxicity against human 5-FU-resistant Patu-T cells by crystal violet staining, IC50 = 33.2 μM. | 20930123 | |||
| Hep2 | Anticancer assay | 48 hrs | Anticancer activity against human Hep2 cells assessed as cell viability after 48 hrs by SRB assay, IC50 = 0.3 μM. | 21074444 | ||
| A549 | Anticancer assay | 48 hrs | Anticancer activity against human A549 cells assessed as cell viability after 48 hrs by SRB assay, IC50 = 2.4 μM. | 21074444 | ||
| HCT15 | Anticancer assay | 48 hrs | Anticancer activity against human HCT15 cells assessed as cell viability after 48 hrs by SRB assay, IC50 = 3.6 μM. | 21074444 | ||
| 502713 | Anticancer assay | 48 hrs | Anticancer activity against human 502713 cells assessed as cell viability after 48 hrs by SRB assay, IC50 = 4.6 μM. | 21074444 | ||
| DU145 | Anticancer assay | 48 hrs | Anticancer activity against human DU145 cells assessed as cell viability after 48 hrs by SRB assay, IC50 = 4.8 μM. | 21074444 | ||
| PC3 | Anticancer assay | 48 hrs | Anticancer activity against human PC3 cells assessed as cell viability after 48 hrs by SRB assay, IC50 = 12 μM. | 21074444 | ||
| IMR32 | Anticancer assay | 48 hrs | Anticancer activity against human IMR32 cells assessed as cell viability after 48 hrs by SRB assay, IC50 = 18 μM. | 21074444 | ||
| MCF7 | Cytotoxicity assay | 3 days | Cytotoxicity against human MCF7 cells expressing wild type p53 and ras mutant after 3 days by SRB assay, IC50 = 4.32 μM. | 21126804 | ||
| KB | Cytotoxicity assay | 48 hrs | Cytotoxicity against human KB cells after 48 hrs by MTT assay, IC50 = 2.16 μM. | 21131104 | ||
| HepG2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 cells after 48 hrs by MTT assay, IC50 = 2.78 μM. | 21131104 | ||
| K562 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human K562 cells after 48 hrs by MTT assay, IC50 = 38 μM. | 21131104 | ||
| KB | Cytotoxicity assay | 3 days | Cytotoxicity against human KB cells after 3 days by MTT assay, IC50 = 11.9 μM. | 21144621 | ||
| MGC803 | Cytotoxicity assay | 3 days | Cytotoxicity against human MGC803 cells after 3 days by MTT assay, IC50 = 12.6 μM. | 21144621 | ||
| CNE2 | Cytotoxicity assay | 3 days | Cytotoxicity against human CNE2 cells after 3 days by MTT assay, IC50 = 13.1 μM. | 21144621 | ||
| MCF7 | Cytotoxicity assay | 3 days | Cytotoxicity against human MCF7 cells after 3 days by MTT assay, IC50 = 15.2 μM. | 21144621 | ||
| L1210 | Cytotoxicity assay | 2 days | Cytotoxicity against mouse L1210 cells after 2 days by coulter counter analysis, IC50 = 0.56 μM. | 21232828 | ||
| HeLa | Cytotoxicity assay | 4 days | Cytotoxicity against human HeLa cells after 4 days by coulter counter analysis, IC50 = 0.57 μM. | 21232828 | ||
| CEM | Cytotoxicity assay | 3 days | Cytotoxicity against human CEM cells after 3 days by coulter counter analysis, IC50 = 14 μM. | 21232828 | ||
| HL60 | Cytotoxicity assay | 48 hrs | Cytotoxicity in human HL60 cells assessed as cell viability after 48 hrs by MTT assay, IC50 = 9.5 μM. | 21250700 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity in human A549 cells assessed as cell viability after 48 hrs by MTT assay, IC50 = 11.3 μM. | 21250700 | ||
| KB | Cytotoxicity assay | 72 hrs | Cytotoxicity against human KB cells after 72 hrs by MTT assay, IC50 = 15.9 μM. | 21273072 | ||
| MDA-MB-231 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MDA-MB-231 cells assessed as growth inhibition after 72 hrs, IC50 = 4.7 μM. | 21295381 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells assessed as growth inhibition after 72 hrs, IC50 = 9.6 μM. | 21295381 | ||
| L1210 | Function assay | 24 hrs | Inhibition of thymidylate synthase in mouse L1210 cells assessed as inhibition of tritium release from [5-3H]deoxyuridine after preincubation for 24 hrs by liquid scintillation counting, IC50 = 0.02 μM. | 21330014 | ||
| L1210 | Function assay | 24 hrs | Inhibition of thymidylate synthase in mouse L1210 cells assessed as inhibition of tritium release from [5-3H]deoxycytidine after preincubation for 24 hrs by liquid scintillation counting, IC50 = 0.06 μM. | 21330014 | ||
| FM3A | Cytostatic activity assay | 2 days | Cytostatic activity against mouse FM3A cells after 2 days by coulter counting analysis, IC50 = 0.18 μM. | 21330014 | ||
| HeLa | Cytostatic activity assay | Cytostatic activity against human HeLa cells in presence of 20 uM 2'-deoxyuridine, IC50 = 0.18 μM. | 21330014 | |||
| L1210 | Function assay | 4 hrs | Inhibition of thymidylate synthase in mouse L1210 cells assessed as inhibition of tritium release from [5-3H]deoxyuridine after preincubation for 4 hrs by liquid scintillation counting, IC50 = 0.22 μM. | 21330014 | ||
| L1210 | Function assay | 24 hrs | Inhibition of DNA synthesis in mouse L1210 cells assessed as [6-3H]2'-deoxyuridine incorporation in to DNA preincubated for 24 hrs by liquid scintillation counting, IC50 = 0.37 μM. | 21330014 | ||
| HeLa | Cytostatic activity assay | 3 days | Cytostatic activity against human HeLa cells after 3 days by coulter counting analysis, IC50 = 0.45 μM. | 21330014 | ||
| L1210 | Cytostatic activity assay | Cytostatic activity against mouse L1210 cells in presence of 500 uM uracil, IC50 = 0.47 μM. | 21330014 | |||
| L1210 | Cytostatic activity assay | 2 days | Cytostatic activity against mouse L1210 cells after 2 days by coulter counting analysis, IC50 = 0.56 μM. | 21330014 | ||
| L1210 | Function assay | 4 hrs | Inhibition of thymidylate synthase in mouse L1210 cells assessed as inhibition of tritium release from [5-3H]deoxycytidine after preincubation for 4 hrs by liquid scintillation counting, IC50 = 0.6 μM. | 21330014 | ||
| L1210 | Cytostatic activity assay | Cytostatic activity against mouse L1210 cells in presence of 500 uM uridine, IC50 = 0.62 μM. | 21330014 | |||
| L1210 | Function assay | Inhibition of DNA synthesis in mouse L1210 cells assessed as [6-3H]2'-deoxyuridine incorporation in to DNA by liquid scintillation counting, IC50 = 0.97 μM. | 21330014 | |||
| HeLa | Cytostatic activity assay | Cytostatic activity against human HeLa cells in presence of 500 uM uracil, IC50 = 1 μM. | 21330014 | |||
| HeLa | Cytostatic activity assay | Cytostatic activity against human HeLa cells in presence of 500 uM uridine, IC50 = 1.1 μM. | 21330014 | |||
| L1210 | Cytostatic activity assay | Cytostatic activity against mouse L1210 cells in presence of 20 uM 2'-deoxyuridine, IC50 = 1.3 μM. | 21330014 | |||
| HeLa | Cytostatic activity assay | Cytostatic activity against human HeLa cells in presence of 20 uM thymidine, IC50 = 1.5 μM. | 21330014 | |||
| L1210 | Function assay | 24 hrs | Inhibition of RNA synthesis in mouse L1210 cells assessed as [5-3H]uridine incorporation in to RNA preincubated for 24 hrs by liquid scintillation counting, IC50 = 4.9 μM. | 21330014 | ||
| L1210 | Function assay | 15 mins | Inhibition of thymidylate synthase in mouse L1210 cells assessed as inhibition of tritium release from [5-3H]deoxyuridine after preincubation for 15 mins by liquid scintillation counting, IC50 = 7 μM. | 21330014 | ||
| CEM | Cytostatic activity assay | 3 days | Cytostatic activity against human CEM cells after 3 days by coulter counting analysis, IC50 = 14 μM. | 21330014 | ||
| L1210 | Cytostatic activity assay | Cytostatic activity against mouse L1210 cells in presence of 20 uM thymidine, IC50 = 21 μM. | 21330014 | |||
| HL60 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HL60 cells assessed as cell viability after 48 hrs by MTT assay, IC50 = 9.1 μM. | 21381696 | ||
| CEM | Cytotoxicity assay | 72 hrs | Cytotoxicity against human CEM cells after 72 hrs by trypan blue assay, IC50 = 8 μM. | 21439690 | ||
| CEM | Cytotoxicity assay | 48 hrs | Cytotoxicity against human CEM cells after 48 hrs by trypan blue assay, IC50 = 15 μM. | 21439690 | ||
| CEM | Cytotoxicity assay | 72 hrs | Cytotoxicity against human CEM cells after 72 hrs by MTT assay, IC50 = 30 μM. | 21439690 | ||
| CEM | Cytotoxicity assay | 48 hrs | Cytotoxicity against human CEM cells after 48 hrs by MTT assay, IC50 = 40 μM. | 21439690 | ||
| K562 | Anticancer assay | Anticancer activity against human K562 cells, IC50 = 2.4 μM. | 21449610 | |||
| COLO205 | Anticancer assay | Anticancer activity against human COLO205 cells, IC50 = 4.17 μM. | 21449610 | |||
| KM12 | Anticancer assay | Anticancer activity against human KM12 cells, IC50 = 8.32 μM. | 21449610 | |||
| HCT116 | Anticancer assay | Anticancer activity against human HCT116 cells, IC50 = 8.51 μM. | 21449610 | |||
| HCT15 | Anticancer assay | Anticancer activity against human HCT15 cells, IC50 = 9.71 μM. | 21449610 | |||
| HT-29 | Anticancer assay | Anticancer activity against human HT-29 cells, IC50 = 10.2 μM. | 21449610 | |||
| HCC2998 | Anticancer assay | Anticancer activity against human HCC2998 cells, IC50 = 13.5 μM. | 21449610 | |||
| CCRF-CEM | Anticancer assay | Anticancer activity against human CCRF-CEM cells, IC50 = 33.1 μM. | 21449610 | |||
| MIAPaCa2 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MIAPaCa2 cells after 72 hrs, IC50 = 7.4 μM. | 21458113 | ||
| COLO320 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human COLO320 cells after 72 hrs, IC50 = 8.7 μM. | 21458113 | ||
| COLO320 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human COLO320 cells after 48 hrs, IC50 = 10 μM. | 21458113 | ||
| MIAPaCa2 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MIAPaCa2 cells after 48 hrs, IC50 = 14 μM. | 21458113 | ||
| MCF7 | Antitumor assay | 72 hrs | Antitumor activity against human MCF7 cells after 72 hrs by MTT assay, IC50 = 7.54 μM. | 21486694 | ||
| EC9706 | Antitumor assay | 72 hrs | Antitumor activity against human EC9706 cells after 72 hrs by MTT assay, IC50 = 20.3 μM. | 21486694 | ||
| SPC-A1 | Antitumor assay | 72 hrs | Antitumor activity against human SPC-A1 cells after 72 hrs by MTT assay, IC50 = 26.92 μM. | 21486694 | ||
| PC3 | Antitumor assay | 72 hrs | Antitumor activity against human PC3 cells after 72 hrs by MTT assay, IC50 = 29.3 μM. | 21486694 | ||
| HeLa | Antitumor assay | 72 hrs | Antitumor activity against human HeLa cells after 72 hrs by MTT assay, IC50 = 41.46 μM. | 21486694 | ||
| ZR-75-1 | Growth inhibition assay | 48 hrs | Growth inhibition of human ZR-75-1 cells after 48 hrs by sulforhodamine B assay, GI50 = 2.3 μM. | 21489802 | ||
| A549 | Growth inhibition assay | 48 hrs | Growth inhibition of human A549 cells after 48 hrs by sulforhodamine B assay, GI50 = 2.4 μM. | 21489802 | ||
| COLO205 | Growth inhibition assay | 48 hrs | Growth inhibition of human COLO205 cells after 48 hrs by sulforhodamine B assay, GI50 = 2.8 μM. | 21489802 | ||
| MCF7 | Growth inhibition assay | 48 hrs | Growth inhibition of human MCF7 cells after 48 hrs by sulforhodamine B assay, GI50 = 2.8 μM. | 21489802 | ||
| Gurav | Growth inhibition assay | 48 hrs | Growth inhibition of human Gurav cells after 48 hrs by sulforhodamine B assay, GI50 = 3.1 μM. | 21489802 | ||
| A2780 | Growth inhibition assay | 48 hrs | Growth inhibition of human A2780 cells after 48 hrs by sulforhodamine B assay, GI50 = 3.5 μM. | 21489802 | ||
| HOP62 | Growth inhibition assay | 48 hrs | Growth inhibition of human HOP62 cells after 48 hrs by sulforhodamine B assay, GI50 = 4.1 μM. | 21489802 | ||
| KB | Growth inhibition assay | 48 hrs | Growth inhibition of human KB cells after 48 hrs by sulforhodamine B assay, GI50 = 5.2 μM. | 21489802 | ||
| DWD | Growth inhibition assay | 48 hrs | Growth inhibition of human DWD cells after 48 hrs by sulforhodamine B assay, GI50 = 6.1 μM. | 21489802 | ||
| SiHa | Growth inhibition assay | 48 hrs | Growth inhibition of human SiHa cells after 48 hrs by sulforhodamine B assay, GI50 = 6.3 μM. | 21489802 | ||
| PC3 | Growth inhibition assay | 48 hrs | Growth inhibition of human PC3 cells after 48 hrs by sulforhodamine B assay, GI50 = 12 μM. | 21489802 | ||
| SMMC7721 | Cytotoxicity assay | Cytotoxicity against human SMMC7721 cells, IC50 = 13 μM. | 21495659 | |||
| A549 | Cytotoxicity assay | Cytotoxicity against human A549 cells, IC50 = 13.7 μM. | 21495659 | |||
| Bel7402 | Cytotoxicity assay | Cytotoxicity against human Bel7402 cells, IC50 = 21.8 μM. | 21495659 | |||
| A549 | Cytotoxicity assay | Cytotoxicity against human A549 cells by MTT assay, IC50 = 5.6 μM. | 21515044 | |||
| QGY | Cytotoxicity assay | Cytotoxicity against human QGY cells by MTT assay, IC50 = 14.1 μM. | 21515044 | |||
| HeLa | Cytotoxicity assay | Cytotoxicity against human HeLa cells by MTT assay, IC50 = 16.1 μM. | 21515044 | |||
| SW620 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SW620 cells after 72 hrs by MTT assay, IC50 = 3.38 μM. | 21524829 | ||
| HeLa | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HeLa cells after 72 hrs by MTT assay, IC50 = 10.31 μM. | 21524829 | ||
| MIAPaCa2 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MIAPaCa2 cells after 72 hrs by MTT assay, IC50 = 11.45 μM. | 21524829 | ||
| WI38 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human WI38 cells after 72 hrs by MTT assay, IC50 = 18.5 μM. | 21524829 | ||
| SKBR3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SKBR3 cells after 72 hrs by MTT assay, IC50 = 38.25 μM. | 21524829 | ||
| MCF7 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MCF7 cells after 72 hrs by MTT assay, IC50 = 42.01 μM. | 21524829 | ||
| PA1 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human PA1 cells after 48 hrs by resazurin reduction test, IC50 = 5 μM. | 21530016 | ||
| MCF7 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MCF7 cells after 48 hrs by resazurin reduction test, IC50 = 15 μM. | 21530016 | ||
| HeLa | Cytotoxicity assay | 3 days | Cytotoxicity against human HeLa cells after 3 days by MTT assay, IC50 = 4 μM. | 21612935 | ||
| MG63 | Cytotoxicity assay | 3 days | Cytotoxicity against human MG63 cells after 3 days by MTT assay, IC50 = 8 μM. | 21612935 | ||
| L1210 | Cytotoxicity assay | 72 hrs | Cytotoxicity against mouse L1210 cells after 72 hrs by MTT assay, IC50 = 0.0000011 μM. | 21640594 | ||
| P388 | Cytotoxicity assay | 72 hrs | Cytotoxicity against mouse P388 cells after 72 hrs by MTT assay, IC50 = 0.0000017 μM. | 21640594 | ||
| HL60 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HL60 cells after 72 hrs by MTT assay, IC50 = 0.0000027 μM. | 21640594 | ||
| KB | Cytotoxicity assay | 72 hrs | Cytotoxicity against human KB cells after 72 hrs by MTT assay, IC50 = 0.0000077 μM. | 21640594 | ||
| IEC-6 | Cytotoxicity assay | 6 days | Cytotoxicity against rat IEC-6 cells after 6 days by SRB colorimetric assay, IC50 = 0.66 μM. | 21684047 | ||
| MCF10A | Cytotoxicity assay | 6 days | Cytotoxicity against human MCF10A cells after 6 days by SRB colorimetric assay, IC50 = 0.73 μM. | 21684047 | ||
| HT-29 | Cytotoxicity assay | 6 days | Cytotoxicity against human HT-29 cells after 6 days by SRB colorimetric assay, IC50 = 1.08 μM. | 21684047 | ||
| MCF7 | Cytotoxicity assay | 6 days | Cytotoxicity against human MCF7 cells after 6 days by SRB colorimetric assay, IC50 = 4.32 μM. | 21684047 | ||
| DU145 | Cytotoxicity assay | Cytotoxicity against human DU145 cells, IC50 = 3.3 μM. | 21774474 | |||
| NCI-H460 | Cytotoxicity assay | Cytotoxicity against human NCI-H460 cells, IC50 = 8.5 μM. | 21774474 | |||
| MCF7 | Cytotoxicity assay | Cytotoxicity against human MCF7 cells, IC50 = 31 μM. | 21774474 | |||
| KB | Cytotoxicity assay | 3 days | Cytotoxicity against human KB cells after 3 days by MTT assay, IC50 = 12.5 μM. | 21843939 | ||
| HeLa | Cytostatic activity assay | 72 hrs | Cytostatic activity against thymidine-kinase deficient human HeLa cells after 72 hrs by cell counting, IC50 = 0.23 μM. | 21892829 | ||
| L1210 | Cytostatic activity assay | 48 hrs | Cytostatic activity against thymidine-kinase deficient mouse L1210 cells after 48 hrs by cell counting, IC50 = 0.32 μM. | 21892829 | ||
| HeLa | Cytostatic activity assay | 72 hrs | Cytostatic activity against human HeLa cells after 72 hrs by cell counting, IC50 = 0.54 μM. | 21892829 | ||
| CEM | Cytostatic activity assay | 72 hrs | Cytostatic activity against thymidine-kinase deficient human CEM cells after 72 hrs by cell counting, IC50 = 12 μM. | 21892829 | ||
| CEM/0 | Cytostatic activity assay | 72 hrs | Cytostatic activity against human CEM/0 cells after 72 hrs by cell counting, IC50 = 18 μM. | 21892829 | ||
| L1210 | Cytostatic activity assay | Cytostatic activity against mouse L1210 cells, IC50 = 0.49 μM. | 21917363 | |||
| HeLa | Cytostatic activity assay | Cytostatic activity against human HeLa cells, IC50 = 0.54 μM. | 21917363 | |||
| CEM | Cytostatic activity assay | Cytostatic activity against human CEM cells, IC50 = 18 μM. | 21917363 | |||
| A549 | Cytotoxicity assay | 3 days | Cytotoxicity against human A549 cells after 3 days by MTT assay, IC50 = 0.42 μM. | 22000925 | ||
| HepG2 | Cytotoxicity assay | 3 days | Cytotoxicity against human HepG2 cells after 3 days by MTT assay, IC50 = 2.7 μM. | 22000925 | ||
| BCG823 | Cytotoxicity assay | 3 days | Cytotoxicity against human BCG823 cells after 3 days by MTT assay, IC50 = 6.15 μM. | 22000925 | ||
| colon cancer cell | Cytotoxicity assay | 48 hrs | Cytotoxicity against human colon cancer cells after 48 hrs by coulter counting, GI50 = 8.4 μM. | 22056277 | ||
| leukemia cell | Cytotoxicity assay | 48 hrs | Cytotoxicity against human leukemia cells after 48 hrs by coulter counting, GI50 = 15.1 μM. | 22056277 | ||
| prostate cancer cell | Cytotoxicity assay | 48 hrs | Cytotoxicity against human prostate cancer cells after 48 hrs by coulter counting, GI50 = 22.7 μM. | 22056277 | ||
| renal cancer cell | Cytotoxicity assay | 48 hrs | Cytotoxicity against human renal cancer cells after 48 hrs by coulter counting, GI50 = 45.6 μM. | 22056277 | ||
| MCF7 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MCF7 cells after 72 hrs by MTT assay, IC50 = 9.67 μM. | 22079378 | ||
| KB | Antiproliferative assay | 72 hrs | Antiproliferative activity against human KB cells after 72 hrs by MTT assay, IC50 = 9.85 μM. | 22079378 | ||
| GLC82 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human GLC82 cells after 72 hrs by MTT assay, IC50 = 15.5 μM. | 22079378 | ||
| MGC803 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MGC803 cells after 72 hrs by MTT assay, IC50 = 16.6 μM. | 22079378 | ||
| CNE2 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human CNE2 cells after 72 hrs by MTT assay, IC50 = 17.2 μM. | 22079378 | ||
| HEK293 | Cytotoxicity assay | 2 days | Cytotoxicity against human HEK293 cells after 2 days by alamar blue assay, IC50 = 42.9 μM. | 22093761 | ||
| colon cancer cell | Growth inhibition assay | 48 hrs | Growth inhibition of human colon cancer cells after 48 hrs by coulter counter method, GI50 = 8.4 μM. | 22119151 | ||
| leukemia cell | Growth inhibition assay | 48 hrs | Growth inhibition of human leukemia cells after 48 hrs by coulter counter method, GI50 = 15.1 μM. | 22119151 | ||
| prostate cancer cell | Growth inhibition assay | 48 hrs | Growth inhibition of human prostate cancer cells after 48 hrs by coulter counter method, GI50 = 22.7 μM. | 22119151 | ||
| renal cancer cell | Growth inhibition assay | 48 hrs | Growth inhibition of human renal cancer cells after 48 hrs by coulter counter method, GI50 = 45.6 μM. | 22119151 | ||
| H460 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human H460 cells after 72 hrs by MTT assay, IC50 = 3 μM. | 22130132 | ||
| HCT116 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT116 cells after 72 hrs by MTT assay, IC50 = 4 μM. | 22130132 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by MTT assay, IC50 = 14 μM. | 22130132 | ||
| A549 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human A549 cells after 24 hrs by MTT assay, IC50 = 5.64 μM. | 22182926 | ||
| QGY7701 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human QGY7701 cells after 24 hrs by MTT assay, IC50 = 14.3 μM. | 22182926 | ||
| SW480 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human SW480 cells after 24 hrs by MTT assay, IC50 = 15.71 μM. | 22182926 | ||
| HeLa | Cytotoxicity assay | 24 hrs | Cytotoxicity against human HeLa cells after 24 hrs by MTT assay, IC50 = 16.32 μM. | 22182926 | ||
| KB | Cytotoxicity assay | 72 hrs | Cytotoxicity against human KB cells after 72 hrs ny MTT assay, IC50 = 9 μM. | 22200404 | ||
| CNE2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human CNE2 cells after 72 hrs ny MTT assay, IC50 = 13.5 μM. | 22200404 | ||
| HepG2 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HepG2 cells after 48 hrs by MTT assay, IC50 = 15.92 μM. | 22209486 | ||
| NCI-H460 | Cytotoxicity assay | Cytotoxicity against human NCI-H460 cells, IC50 = 8 μM. | 22283451 | |||
| SMMC7721 | Cytotoxicity assay | Cytotoxicity against human SMMC7721 cells, IC50 = 15 μM. | 22283451 | |||
| MCF7 | Cytotoxicity assay | Cytotoxicity against human MCF7 cells, IC50 = 31 μM. | 22283451 | |||
| NCI-H460 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human NCI-H460 cells after 48 hrs by MTT assay, IC50 = 29 μM. | 22304344 | ||
| B16 | Cytotoxicity assay | 48 hrs | Cytotoxicity against mouse B16 cells after 48 hrs by MTT assay, IC50 = 33 μM. | 22304344 | ||
| HepG2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 cells after 48 hrs by MTT assay, IC50 = 34 μM. | 22304344 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MCF7 cells after 48 hrs by MTT assay, IC50 = 35 μM. | 22304344 | ||
| HeLa | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HeLa cells after 48 hrs by MTT assay, IC50 = 43 μM. | 22304344 | ||
| SW1990 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human SW1990 cells after 48 hrs by MTT assay, IC50 = 45 μM. | 22304344 | ||
| SR | Cytotoxicity assay | 48 hrs | Cytotoxicity against human SR cells after 48 hrs by SRB assay, GI50 = 0.0238 μM. | 22305543 | ||
| NCI-H522 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human NCI-H522 cells after 48 hrs by SRB assay, GI50 = 7.28 μM. | 22305543 | ||
| WHCO1 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human WHCO1 cells after 48 hrs by MTT assay, IC50 = 7.9 μM. | 22381354 | ||
| HaCaT | Cytostatic activity assay | 72 hrs | Cytostatic activity against human HaCaT cells after 72 hrs by MTT assay, IC50 = 0.3 μM. | 22405290 | ||
| L1210 | Cytostatic activity assay | 2 days | Cytostatic activity against mouse L1210 cells after 2 days by coulter counter analysis, IC50 = 0.49 μM. | 22405290 | ||
| HeLa | Cytostatic activity assay | 3 days | Cytostatic activity against human HeLa cells after 3 days by coulter counter analysis, IC50 = 0.54 μM. | 22405290 | ||
| NCI-H460 | Cytostatic activity assay | 72 hrs | Cytostatic activity against human NCI-H460 cells after 72 hrs by MTT assay, IC50 = 3 μM. | 22405290 | ||
| HCT116 | Cytostatic activity assay | 72 hrs | Cytostatic activity against human HCT116 cells after 72 hrs by MTT assay, IC50 = 4 μM. | 22405290 | ||
| MCF7 | Cytostatic activity assay | 72 hrs | Cytostatic activity against human MCF7 cells after 72 hrs by MTT assay, IC50 = 15 μM. | 22405290 | ||
| CEM | Cytostatic activity assay | 3 days | Cytostatic activity against human CEM cells after 3 days by coulter counter analysis, IC50 = 18 μM. | 22405290 | ||
| MGC803 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MGC803 cells after 48 hrs by MTT assay, IC50 = 3.42 μM. | 22405648 | ||
| Bcap37 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human Bcap37 cells after 48 hrs by MTT assay, IC50 = 5.33 μM. | 22405648 | ||
| SGC7901 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human SGC7901 cells after 48 hrs by MTT assay, IC50 = 7.11 μM. | 22405648 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by SRB assay, IC50 = 3.5 μM. | 22409771 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells after 72 hrs by SRB assay, IC50 = 5 μM. | 22409771 | ||
| HCT116 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT116 cells after 72 hrs by SRB assay, IC50 = 6 μM. | 22409771 | ||
| FOCUS | Cytotoxicity assay | 72 hrs | Cytotoxicity against human FOCUS cells after 72 hrs by SRB assay, IC50 = 7.6 μM. | 22409771 | ||
| Mahlavu | Cytotoxicity assay | 72 hrs | Cytotoxicity against human Mahlavu cells after 72 hrs by SRB assay, IC50 = 10 μM. | 22409771 | ||
| HuH7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HuH7 cells after 72 hrs by SRB assay, IC50 = 30.7 μM. | 22409771 | ||
| K562 | Cytotoxicity assay | Cytotoxicity against human K562 cells, IC50 = 2.4 μM. | 22409967 | |||
| COLO205 | Cytotoxicity assay | Cytotoxicity against human COLO205 cells, IC50 = 4.17 μM. | 22409967 | |||
| KM12 | Cytotoxicity assay | Cytotoxicity against human KM12 cells, IC50 = 8.32 μM. | 22409967 | |||
| HCT116 | Cytotoxicity assay | Cytotoxicity against human HCT116 cells, IC50 = 8.51 μM. | 22409967 | |||
| HCT15 | Cytotoxicity assay | Cytotoxicity against human HCT15 cells, IC50 = 9.71 μM. | 22409967 | |||
| HT-29 | Cytotoxicity assay | Cytotoxicity against human HT-29 cells, IC50 = 10.2 μM. | 22409967 | |||
| HCC2998 | Cytotoxicity assay | Cytotoxicity against human HCC2998 cells, IC50 = 13.5 μM. | 22409967 | |||
| PC3 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human PC3 cells after 48 hrs by sulforhodamine B assay, IC50 = 2.1 μM. | 22424614 | ||
| MOLT4 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MOLT4 cells after 48 hrs by sulforhodamine B assay, IC50 = 3.2 μM. | 22424614 | ||
| MCF7 | Antiproliferative assay | 144 hrs | Antiproliferative activity against human MCF7 cells assessed as growth inhibition after 144 hrs by crystal violet staining method, IC50 = 4.7 μM. | 22450132 | ||
| HT-29 | Antiproliferative assay | 144 hrs | Antiproliferative activity against human HT-29 cells assessed as growth inhibition after 144 hrs by crystal violet staining method, IC50 = 7.3 μM. | 22450132 | ||
| MDA-MB-231 | Antiproliferative assay | 144 hrs | Antiproliferative activity against human MDA-MB-231 cells assessed as growth inhibition after 144 hrs by crystal violet staining method, IC50 = 9.6 μM. | 22450132 | ||
| PC9 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human PC9 cells after 48 hrs by MTT assay, IC50 = 23.47 μM. | 22483608 | ||
| HCT116 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HCT116 cells after 48 hrs by MTT assay, IC50 = 31.72 μM. | 22483608 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells after 48 hrs by MTT assay, IC50 = 35.05 μM. | 22483608 | ||
| HepG2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 cells after 48 hrs by MTT assay, IC50 = 46.31 μM. | 22483608 | ||
| A375 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A375 cells after 48 hrs by MTT assay, IC50 = 46.55 μM. | 22483608 | ||
| SKHEP1 | Growth inhibition assay | 48 hrs | Growth inhibition of human SKHEP1 cells after 48 hrs by SRB assay, GI50 = 6.3 μM. | 22512908 | ||
| NCI-H460 | Growth inhibition assay | 48 hrs | Growth inhibition of human NCI-H460 cells after 48 hrs by SRB assay, GI50 = 6.5 μM. | 22512908 | ||
| BxPC3 | Growth inhibition assay | 48 hrs | Growth inhibition of human BxPC3 cells after 48 hrs by SRB assay, GI50 = 8.2 μM. | 22512908 | ||
| MSC | Growth inhibition assay | 48 hrs | Growth inhibition of human MSC cells after 48 hrs by SRB assay, GI50 = 8.92 μM. | 22512908 | ||
| IGROV1 | Growth inhibition assay | 48 hrs | Growth inhibition of human IGROV1 cells after 48 hrs by SRB assay, GI50 = 9.1 μM. | 22512908 | ||
| DU145 | Growth inhibition assay | 48 hrs | Growth inhibition of human DU145 cells after 48 hrs by SRB assay, GI50 = 9.37 μM. | 22512908 | ||
| HUVEC | Growth inhibition assay | 48 hrs | Growth inhibition of HUVEC cells after 48 hrs by SRB assay, GI50 = 9.72 μM. | 22512908 | ||
| HCT116 | Growth inhibition assay | 48 hrs | Growth inhibition of human HCT116 cells after 48 hrs by SRB assay, GI50 = 9.9 μM. | 22512908 | ||
| MCF7 | Growth inhibition assay | 48 hrs | Growth inhibition of human MCF7 cells after 48 hrs by SRB assay, GI50 = 9.9 μM. | 22512908 | ||
| OVCAR5 | Growth inhibition assay | 48 hrs | Growth inhibition of human OVCAR5 cells after 48 hrs by SRB assay, GI50 = 20 μM. | 22512908 | ||
| HCT15 | Growth inhibition assay | 48 hrs | Growth inhibition of human HCT15 cells after 48 hrs by SRB assay, GI50 = 21 μM. | 22512908 | ||
| PC3 | Growth inhibition assay | 48 hrs | Growth inhibition of human PC3 cells after 48 hrs by SRB assay, GI50 = 21 μM. | 22512908 | ||
| MIAPaCa2 | Growth inhibition assay | 48 hrs | Growth inhibition of human MIAPaCa2 cells after 48 hrs by SRB assay, GI50 = 30 μM. | 22512908 | ||
| K562 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human K562 cells after 48 hrs by MTT assay, IC50 = 2.1 μM. | 22537362 | ||
| KB | Cytotoxicity assay | 48 hrs | Cytotoxicity against human KB cells after 48 hrs by MTT assay, IC50 = 6.8 μM. | 22537362 | ||
| KB/VCR | Cytotoxicity assay | 48 hrs | Cytotoxicity against human KB/VCR cells after 48 hrs by MTT assay, IC50 = 33.4 μM. | 22537362 | ||
| SW620 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SW620 cells after 72 hrs by MTT assay, IC50 = 6.68 μM. | 22555152 | ||
| MIAPaCa2 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MIAPaCa2 cells after 72 hrs by MTT assay, IC50 = 11.67 μM. | 22555152 | ||
| HeLa | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HeLa cells after 72 hrs by MTT assay, IC50 = 14.93 μM. | 22555152 | ||
| WI38 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human WI38 cells after 72 hrs by MTT assay, IC50 = 24.66 μM. | 22555152 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells by after 48 hrs by MTT assay, IC50 = 5.64 μM. | 22579780 | ||
| SGC7901 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human SGC7901 cells by after 48 hrs by MTT assay, IC50 = 6.04 μM. | 22579780 | ||
| MDA-MB-231 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MDA-MB-231 cells by after 48 hrs by MTT assay, IC50 = 13.65 μM. | 22579780 | ||
| QGY7701 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human QGY7701 cells by after 48 hrs by MTT assay, IC50 = 14.3 μM. | 22579780 | ||
| SW480 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human SW480 cells by after 48 hrs by MTT assay, IC50 = 15.71 μM. | 22579780 | ||
| HeLa | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HeLa cells by after 48 hrs by MTT assay, IC50 = 16.32 μM. | 22579780 | ||
| L02 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human L02 cells by after 48 hrs by MTT assay, IC50 = 46.57 μM. | 22579780 | ||
| SH-SY5Y | Cytotoxicity assay | 72 hrs | Cytotoxicity against human SH-SY5Y cells after 72 hrs by MTT assay, IC50 = 1.09 μM. | 22687745 | ||
| L929 | Cytotoxicity assay | 72 hrs | Cytotoxicity against mouse L929 cells after 72 hrs by MTT assay, IC50 = 1.38 μM. | 22687745 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells after 72 hrs by MTT assay, IC50 = 8.83 μM. | 22687745 | ||
| DU145 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human DU145 cells after 72 hrs by MTT assay, IC50 = 12.32 μM. | 22687745 | ||
| SGC7901 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human SGC7901 cells after 72 hrs by MTT assay, IC50 = 14.49 μM. | 22687745 | ||
| HeLa | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HeLa cells after 72 hrs by MTT assay, IC50 = 15.99 μM. | 22687745 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells after 72 hrs by MTT assay, IC50 = 32.22 μM. | 22687745 | ||
| NCI-H460 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human NCI-H460 cells after 72 hrs by MTT assay, IC50 = 2.4 μM. | 22727445 | ||
| BGC823 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human BGC823 cells after 72 hrs by MTT assay, IC50 = 4.5 μM. | 22727445 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells after 72 hrs by MTT assay, IC50 = 4.8 μM. | 22727445 | ||
| SGC7901 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human SGC7901 cells after 72 hrs by MTT assay, IC50 = 6.3 μM. | 22727445 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by MTT assay, IC50 = 12.5 μM. | 22727445 | ||
| KB | Cytotoxicity assay | 72 hrs | Cytotoxicity against human KB cells after 72 hrs by MTT assay, IC50 = 8.28 μM. | 22749192 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by MTT assay, IC50 = 14.2 μM. | 22749192 | ||
| CNE2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human CNE2 cells after 72 hrs by MTT assay, IC50 = 15.1 μM. | 22749192 | ||
| GLC82 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human GLC82 cells after 72 hrs by MTT assay, IC50 = 17.4 μM. | 22749192 | ||
| MGC803 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MGC803 cells after 72 hrs by MTT assay, IC50 = 18.5 μM. | 22749192 | ||
| MCF7 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MCF7 cells after 72 hrs by crystal violet staining method, IC50 = 4.7 μM. | 22765897 | ||
| HT-29 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HT-29 cells after 72 hrs by crystal violet staining method, IC50 = 7.3 μM. | 22765897 | ||
| MDA-MB-231 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MDA-MB-231 cells after 72 hrs by crystal violet staining method, IC50 = 9.6 μM. | 22765897 | ||
| HT1080 | Cytotoxicity assay | Cytotoxicity against human HT1080 cells by MTT assay, IC50 = 3.06 μM. | 22801644 | |||
| A375-S2 | Cytotoxicity assay | Cytotoxicity against human A375-S2 cells by MTT assay, IC50 = 9.92 μM. | 22801644 | |||
| HeLa | Cytotoxicity assay | Cytotoxicity against human HeLa cells by MTT assay, IC50 = 10.36 μM. | 22801644 | |||
| HepG2 | Cytotoxicity assay | Cytotoxicity against human HepG2 cells by MTT assay, IC50 = 10.45 μM. | 22801644 | |||
| BGC823 | Cytotoxicity assay | Cytotoxicity against human BGC823 cells by MTT assay, IC50 = 15.65 μM. | 22801644 | |||
| Hep2 | Cytotoxicity assay | Cytotoxicity against human Hep2 cells by MTT assay, IC50 = 24.58 μM. | 22801644 | |||
| MCF7 | Cytotoxicity assay | Cytotoxicity against human MCF7 cells by MTT assay, IC50 = 24.58 μM. | 22801644 | |||
| A549 | Cytotoxicity assay | Cytotoxicity against human A549 cells by MTT assay, IC50 = 31.52 μM. | 22801644 | |||
| MDA231 | Cytotoxicity assay | Cytotoxicity against human MDA231 cells by MTT assay, IC50 = 31.57 μM. | 22801644 | |||
| PC3 | Cytotoxicity assay | Cytotoxicity against human PC3 cells by MTT assay, IC50 = 35.75 μM. | 22801644 | |||
| PC3M | Cytotoxicity assay | 48 hrs | Cytotoxicity against human PC3M cells after 48 hrs by MTT assay, IC50 = 22.63 μM. | 22809558 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells after 48 hrs by MTT assay, IC50 = 35.41 μM. | 22809558 | ||
| MKN45 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MKN45 cells after 48 hrs by MTT assay, IC50 = 41.73 μM. | 22809558 | ||
| HepG2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 cells after 48 hrs by MTT assay, IC50 = 46.83 μM. | 22809558 | ||
| LoVo | Antiproliferative assay | 48 hrs | Antiproliferative activity against human LoVo cells after 48 hrs by MTT assay, IC50 = 3.3 μM. | 22840493 | ||
| A549 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human A549 cells after 48 hrs by MTT assay, IC50 = 12.8 μM. | 22840493 | ||
| MCF7 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MCF7 cells after 48 hrs by MTT assay, IC50 = 16.2 μM. | 22840493 | ||
| IOSE-144 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human IOSE-144 cells after 48 hrs by MTT assay, IC50 = 25.6 μM. | 22840493 | ||
| CAMA1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human CAMA1 cells incubated for 72 hrs by SRB assay, IC50 = 1.28 μM. | 22867707 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells incubated for 72 hrs by SRB assay, IC50 = 3.5 μM. | 22867707 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells incubated for 72 hrs by SRB assay, IC50 = 5.7 μM. | 22867707 | ||
| FOCUS | Cytotoxicity assay | 72 hrs | Cytotoxicity against human FOCUS cells incubated for 72 hrs by SRB assay, IC50 = 7.69 μM. | 22867707 | ||
| T47D | Cytotoxicity assay | 72 hrs | Cytotoxicity against human T47D cells incubated for 72 hrs by SRB assay, IC50 = 8.91 μM. | 22867707 | ||
| MV | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MV cells incubated for 72 hrs by SRB assay, IC50 = 9.97 μM. | 22867707 | ||
| HCT116 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT116 cells incubated for 72 hrs by SRB assay, IC50 = 18.7 μM. | 22867707 | ||
| MFE296 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MFE296 cells incubated for 72 hrs by SRB assay, IC50 = 30.68 μM. | 22867707 | ||
| HuH7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HuH7 cells incubated for 72 hrs by SRB assay, IC50 = 30.7 μM. | 22867707 | ||
| BT20 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human BT20 cells incubated for 72 hrs by SRB assay, IC50 = 47.3 μM. | 22867707 | ||
| COLO320 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human COLO320 cells assessed as cell viability after 72 hrs by WST-1 assay, IC50 = 12.5 μM. | 22955095 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by crystal violet staining, IC50 = 4.7 μM. | 22959205 | ||
| HT-29 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HT-29 cells after 72 hrs by crystal violet staining, IC50 = 7.3 μM. | 22959205 | ||
| MDA-MB-231 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MDA-MB-231 cells after 72 hrs by crystal violet staining, IC50 = 9.6 μM. | 22959205 | ||
| HL60 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HL60 cells after 72 hrs by MTT assay, IC50 = 0.06 μM. | 22959518 | ||
| HeLa | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HeLa cells after 72 hrs by MTT assay, IC50 = 0.28 μM. | 22959518 | ||
| U937 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human U937 cells after 72 hrs by MTT assay, IC50 = 1.07 μM. | 22959518 | ||
| colon cancer | Antiproliferative assay | 48 hrs | Antiproliferative activity against human colon cancer cells after 48 hrs by cell counting analysis, GI50 = 8.4 μM. | 22995621 | ||
| leukemia | Antiproliferative assay | 48 hrs | Antiproliferative activity against human leukemia cells after 48 hrs by cell counting analysis, GI50 = 15.1 μM. | 22995621 | ||
| prostate cancer | Antiproliferative assay | 48 hrs | Antiproliferative activity against human prostate cancer cells after 48 hrs by cell counting analysis, GI50 = 22.7 μM. | 22995621 | ||
| renal cancer | Antiproliferative assay | 48 hrs | Antiproliferative activity against human renal cancer cells after 48 hrs by cell counting analysis, GI50 = 45.6 μM. | 22995621 | ||
| HeLa | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HeLa cells after 48 hrs by MTT assay, IC50 = 9.5 μM. | 23002924 | ||
| MNK45 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MNK45 cells after 48 hrs by MTT assay, IC50 = 12 μM. | 23002924 | ||
| HepG2 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HepG2 cells after 48 hrs by MTT method, IC50 = 1.3 μM. | 23018096 | ||
| BGC823 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human BGC823 cells after 48 hrs by MTT method, IC50 = 1.52 μM. | 23018096 | ||
| SW1116 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human SW1116 cells after 48 hrs by MTT method, IC50 = 2.41 μM. | 23018096 | ||
| HeLa | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HeLa cells after 48 hrs by MTT method, IC50 = 3.25 μM. | 23018096 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells assessed cell viability after 48 hrs by SRB assay, IC50 = 4.21 μM. | 23044370 | ||
| HT1080 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HT1080 cells after 48 hrs by MTT assay, IC50 = 15.2 μM. | 23067549 | ||
| HCT116 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HCT116 cells after 48 hrs by MTT assay, IC50 = 36.4 μM. | 23067549 | ||
| 2008 | Growth inhibition assay | Growth inhibition of human 2008 cells by crystal violet assay, IC50 = 4.2 μM. | 23075414 | |||
| Vero | Growth inhibition assay | Growth inhibition of african green monkey Vero cells by crystal violet assay, IC50 = 4.5 μM. | 23075414 | |||
| A2780 | Growth inhibition assay | Growth inhibition of human A2780 cells by crystal violet assay, IC50 = 5.1 μM. | 23075414 | |||
| C13 | Growth inhibition assay | Growth inhibition of cDDP-resistant human C13 cells by crystal violet assay, IC50 = 8.5 μM. | 23075414 | |||
| A2780/CP70 | Growth inhibition assay | Growth inhibition of cDDP-resistant human A2780/CP70 cells by crystal violet assay, IC50 = 12.3 μM. | 23075414 | |||
| MSC | Growth inhibition assay | 48 hrs | Growth inhibition of human MSC cells after 48 hrs by SRB assay, GI50 = 0.001 μM. | 23094992 | ||
| MSC | Growth inhibition assay | 48 hrs | Growth inhibition of human MSC cells after 48 hrs by SRB assay, TGI = 0.001 μM. | 23094992 | ||
| BxPC3 | Growth inhibition assay | 48 hrs | Growth inhibition of human BxPC3 cells after 48 hrs by SRB assay, GI50 = 0.0029 μM. | 23094992 | ||
| HUVEC | Growth inhibition assay | 48 hrs | Growth inhibition of human HUVEC cells after 48 hrs by SRB assay, GI50 = 0.0036 μM. | 23094992 | ||
| SKHEP1 | Growth inhibition assay | 48 hrs | Growth inhibition of human SKHEP1 cells after 48 hrs by SRB assay, GI50 = 0.0076 μM. | 23094992 | ||
| IGROV1 | Growth inhibition assay | 48 hrs | Growth inhibition of human IGROV1 cells after 48 hrs by SRB assay, GI50 = 0.00812 μM. | 23094992 | ||
| BxPC3 | Growth inhibition assay | 48 hrs | Growth inhibition of human BxPC3 cells after 48 hrs by SRB assay, TGI = 0.0084 μM. | 23094992 | ||
| HCT116 | Growth inhibition assay | 48 hrs | Growth inhibition of human HCT116 cells after 48 hrs by SRB assay, GI50 = 0.0088 μM. | 23094992 | ||
| PC3 | Growth inhibition assay | 48 hrs | Growth inhibition of human PC3 cells after 48 hrs by SRB assay, GI50 = 0.01045 μM. | 23094992 | ||
| MCF7 | Growth inhibition assay | 48 hrs | Growth inhibition of human MCF7 cells after 48 hrs by SRB assay, GI50 = 0.0145 μM. | 23094992 | ||
| DU145 | Growth inhibition assay | 48 hrs | Growth inhibition of human DU145 cells after 48 hrs by SRB assay, GI50 = 0.02 μM. | 23094992 | ||
| HCT15 | Growth inhibition assay | 48 hrs | Growth inhibition of human HCT15 cells after 48 hrs by SRB assay, GI50 = 0.0211 μM. | 23094992 | ||
| OVCAR5 | Growth inhibition assay | 48 hrs | Growth inhibition of human OVCAR5 cells after 48 hrs by SRB assay, GI50 = 0.0264 μM. | 23094992 | ||
| MIAPaCa2 | Growth inhibition assay | 48 hrs | Growth inhibition of human MIAPaCa2 cells after 48 hrs by SRB assay, GI50 = 0.0306 μM. | 23094992 | ||
| NHDF | Growth inhibition assay | 48 hrs | Growth inhibition of human NHDF cells after 48 hrs by SRB assay, GI50 = 0.06202 μM. | 23094992 | ||
| U251 | Growth inhibition assay | 48 hrs | Growth inhibition of human U251 cells after 48 hrs by SRB assay, GI50 = 0.0696 μM. | 23094992 | ||
| NHDF | Growth inhibition assay | 48 hrs | Growth inhibition of human NHDF cells after 48 hrs by SRB assay, TGI = 0.1 μM. | 23094992 | ||
| MIAPaCa2 | Growth inhibition assay | 48 hrs | Growth inhibition of human MIAPaCa2 cells after 48 hrs by SRB assay, TGI = 0.1 μM. | 23094992 | ||
| HUVEC | Growth inhibition assay | 48 hrs | Growth inhibition of human HUVEC cells after 48 hrs by SRB assay, TGI = 0.1 μM. | 23094992 | ||
| OVCAR5 | Growth inhibition assay | 48 hrs | Growth inhibition of human OVCAR5 cells after 48 hrs by SRB assay, TGI = 0.1 μM. | 23094992 | ||
| HL-60(TB) | Growth inhibition assay | 48 hrs | Growth inhibition of human HL-60(TB) cells after 48 hrs by SRB assay, GI50 = 0.1 μM. | 23094992 | ||
| HL-60(TB) | Growth inhibition assay | 48 hrs | Growth inhibition of human HL-60(TB) cells after 48 hrs by SRB assay, TGI = 0.1 μM. | 23094992 | ||
| U251 | Growth inhibition assay | 48 hrs | Growth inhibition of human U251 cells after 48 hrs by SRB assay, TGI = 0.1 μM. | 23094992 | ||
| DU145 | Growth inhibition assay | 48 hrs | Growth inhibition of human DU145 cells after 48 hrs by SRB assay, TGI = 0.1 μM. | 23094992 | ||
| SKHEP1 | Growth inhibition assay | 48 hrs | Growth inhibition of human SKHEP1 cells after 48 hrs by SRB assay, TGI = 0.1 μM. | 23094992 | ||
| MCF7 | Growth inhibition assay | 48 hrs | Growth inhibition of human MCF7 cells after 48 hrs by SRB assay, TGI = 0.1 μM. | 23094992 | ||
| HCT15 | Growth inhibition assay | 48 hrs | Growth inhibition of human HCT15 cells after 48 hrs by SRB assay, TGI = 0.1 μM. | 23094992 | ||
| IGROV1 | Growth inhibition assay | 48 hrs | Growth inhibition of human IGROV1 cells after 48 hrs by SRB assay, TGI = 0.1 μM. | 23094992 | ||
| PC3 | Growth inhibition assay | 48 hrs | Growth inhibition of human PC3 cells after 48 hrs by SRB assay, TGI = 0.1 μM. | 23094992 | ||
| HCT116 | Growth inhibition assay | 48 hrs | Growth inhibition of human HCT116 cells after 48 hrs by SRB assay, TGI = 0.1 μM. | 23094992 | ||
| A549 | Cytotoxicity assay | Cytotoxicity against human A549 cells by MTT method, IC50 = 1.54 μM. | 23164710 | |||
| BGC823 | Cytotoxicity assay | Cytotoxicity against human BGC823 cells by MTT method, IC50 = 3.84 μM. | 23164710 | |||
| Bel7042 | Cytotoxicity assay | Cytotoxicity against human Bel7042 cells by MTT method, IC50 = 3.85 μM. | 23164710 | |||
| HCT8 | Cytotoxicity assay | Cytotoxicity against human HCT8 cells by MTT method, IC50 = 5.38 μM. | 23164710 | |||
| A2780 | Cytotoxicity assay | Cytotoxicity against human A2780 cells by MTT method, IC50 = 5.4 μM. | 23164710 | |||
| NIH-H460 | Cytotoxicity assay | Cytotoxicity against human NIH-H460 cells by MTT method, IC50 = 7.12 μM. | 23164710 | |||
| HepG2 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human HepG2 cells after 24 hrs by MTT assay, IC50 = 29.87 μM. | 23202484 | ||
| PA1 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human PA1 cells after 24 hrs by MTT assay, IC50 = 39.5 μM. | 23202484 | ||
| HCT15 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human HCT15 cells after 24 hrs by MTT assay, IC50 = 45.01 μM. | 23202484 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by MTT assay, IC50 = 17.2 μM. | 23279864 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells after 72 hrs by MTT assay, IC50 = 21.9 μM. | 23279864 | ||
| SGC7901 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human SGC7901 cells after 72 hrs by MTT assay, IC50 = 28.5 μM. | 23279864 | ||
| HCT116 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HCT116 cells assessed as inhibition cell proliferation after 48 hrs by Sulforhodamine B assay, IC50 = 3.56 μM. | 23305919 | ||
| B16F10 | Anticancer assay | 48 hrs | Anticancer activity against mouse B16F10 cells assessed as cell survival measured after 48 hrs by MTT assay, IC50 = 21.41 μM. | 23312949 | ||
| SGC7901 | Anticancer assay | 48 hrs | Anticancer activity against human SGC7901 cells assessed as cell survival measured after 48 hrs by MTT assay, IC50 = 46.35 μM. | 23312949 | ||
| HeLa | Antitumor assay | 48 hrs | Antitumor activity against human HeLa cells assessed as growth inhibition measured after 48 hrs by MTT assay, IC50 = 14.78 μM. | 23313635 | ||
| A549 | Growth inhibition assay | 48 hrs | Growth inhibition of human A549 cells after 48 hrs by MTT assay, IC50 = 5.64 μM. | 23353737 | ||
| QGY7701 | Growth inhibition assay | 48 hrs | Growth inhibition of human QGY7701 cells after 48 hrs by MTT assay, IC50 = 14.3 μM. | 23353737 | ||
| HuH7 | Growth inhibition assay | 48 hrs | Growth inhibition of human HuH7 cells after 48 hrs by MTT assay, IC50 = 15.85 μM. | 23353737 | ||
| HeLa | Growth inhibition assay | 48 hrs | Growth inhibition of human HeLa cells after 48 hrs by MTT assay, IC50 = 16.32 μM. | 23353737 | ||
| L02 | Growth inhibition assay | 48 hrs | Growth inhibition of human L02 cells after 48 hrs by MTT assay, IC50 = 46.57 μM. | 23353737 | ||
| EC109 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human EC109 cells assessed as growth inhibition after 72 hrs by MTT assay, IC50 = 3.34 μM. | 23353743 | ||
| MGC803 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MGC803 cells assessed as growth inhibition after 72 hrs by MTT assay, IC50 = 7.01 μM. | 23353743 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells assessed as growth inhibition after 72 hrs by MTT assay, IC50 = 7.54 μM. | 23353743 | ||
| PC3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human PC3 cells assessed as growth inhibition after 72 hrs by MTT assay, IC50 = 27.07 μM. | 23353743 | ||
| colon cancer | Cytotoxicity assay | 48 hrs | Cytotoxicity against human colon cancer cells after 48 hrs by sulphorhodamine B assay, GI50 = 8.4 μM. | 23474899 | ||
| leukemia | Cytotoxicity assay | 48 hrs | Cytotoxicity against human leukemia cells after 48 hrs by sulphorhodamine B assay, GI50 = 15.1 μM. | 23474899 | ||
| prostate cancer | Cytotoxicity assay | 48 hrs | Cytotoxicity against human prostate cancer cells after 48 hrs by sulphorhodamine B assay, GI50 = 22.7 μM. | 23474899 | ||
| renal cancer | Cytotoxicity assay | 48 hrs | Cytotoxicity against human renal cancer cells after 48 hrs by sulphorhodamine B assay, GI50 = 45.6 μM. | 23474899 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MCF7 cells after 48 hrs by MTT assay, IC50 = 16.7 μM. | 23490159 | ||
| HepG2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 cells after 48 hrs by MTT assay, IC50 = 22.8 μM. | 23490159 | ||
| SGC7901 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human SGC7901 cells after 48 hrs by MTT assay, IC50 = 28.9 μM. | 23490159 | ||
| THP1 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human THP1 cells after 48 hrs by sulforhodamine B assay, IC50 = 1 μM. | 23501113 | ||
| PC3 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human PC3 cells after 48 hrs by sulforhodamine B assay, IC50 = 2.2 μM. | 23501113 | ||
| Hep2 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human Hep2 cells after 48 hrs by sulforhodamine B assay, IC50 = 4.5 μM. | 23501113 | ||
| A549 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human A549 cells after 48 hrs by sulforhodamine B assay, IC50 = 4.9 μM. | 23501113 | ||
| K562 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human K562 cells after 72 hrs by MTT assay, IC50 = 28 μM. | 23535322 | ||
| CEM | Cytotoxicity assay | 72 hrs | Cytotoxicity against human CEM cells after 72 hrs by MTT assay, IC50 = 32 μM. | 23535322 | ||
| A375-S2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A375-S2 cells after 48 hrs by MTT assay, IC50 = 6.67 μM. | 23558238 | ||
| HeLa | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HeLa cells after 48 hrs by MTT assay, IC50 = 7.34 μM. | 23558238 | ||
| HL60 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HL60 cells after 48 hrs by MTT assay, IC50 = 10.05 μM. | 23558238 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MCF7 cells after 48 hrs by MTT assay, IC50 = 11.5 μM. | 23558238 | ||
| K562 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human K562 cells after 48 hrs by MTT assay, IC50 = 23.55 μM. | 23558238 | ||
| U937 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human U937 cells after 48 hrs by MTT assay, IC50 = 23.93 μM. | 23558238 | ||
| HCT116 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HCT116 cells after 48 hrs by MTT assay, IC50 = 27.76 μM. | 23558238 | ||
| HT1080 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HT1080 cells after 48 hrs by MTT assay, IC50 = 35.62 μM. | 23558238 | ||
| HepG2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 cells after 48 hrs by MTT assay, IC50 = 40.34 μM. | 23558238 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells after 48 hrs by MTT assay, IC50 = 44.05 μM. | 23558238 | ||
| EC9706 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human EC9706 cells after 72 hrs by MTT assay, IC50 = 0.32 μM. | 23644193 | ||
| MGC803 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MGC803 cells after 72 hrs by MTT assay, IC50 = 7.14 μM. | 23644193 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by MTT assay, IC50 = 7.33 μM. | 23644193 | ||
| SMMC7721 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human SMMC7721 cells after 72 hrs by MTT assay, IC50 = 26.71 μM. | 23644193 | ||
| HCT116 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HCT116 cells after 48 hrs by sulforhodamine B assay, IC50 = 4.52 μM. | 23644215 | ||
| HT-29 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HT-29 cells after 48 hrs by sulforhodamine B assay, IC50 = 6.35 μM. | 23644215 | ||
| A549 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A549 cells after 72 hrs by MTT assay, IC50 = 3.52 μM. | 23665106 | ||
| HCT116 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT116 cells after 72 hrs by MTT assay, IC50 = 5.53 μM. | 23665106 | ||
| HT-29 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HT-29 cells after 72 hrs by MTT assay, IC50 = 24.5 μM. | 23665106 | ||
| MCF7 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MCF7 cells after 72 hrs by MTT assay, IC50 = 32.18 μM. | 23665106 | ||
| HeLa | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HeLa cells after 72 hrs by MTT assay, IC50 = 43.71 μM. | 23665106 | ||
| colon cancer | Cytotoxicity assay | 48 hrs | Cytotoxicity against human colon cancer cells after 48 hrs by coulter counter analysis, GI50 = 8.4 μM. | 23683592 | ||
| leukemia | Cytotoxicity assay | 48 hrs | Cytotoxicity against human leukemia cells after 48 hrs by coulter counter analysis, GI50 = 15.1 μM. | 23683592 | ||
| prostate cancer | Cytotoxicity assay | 48 hrs | Cytotoxicity against human prostate cancer cells after 48 hrs by coulter counter analysis, GI50 = 22.7 μM. | 23683592 | ||
| renal cancer | Cytotoxicity assay | 48 hrs | Cytotoxicity against human renal cancer cells after 48 hrs by coulter counter analysis, GI50 = 45.6 μM. | 23683592 | ||
| SH-SY5Y | Cytotoxicity assay | 72 hrs | Cytotoxicity against human SH-SY5Y cells after 72 hrs by MTT assay, IC50 = 0.97 μM. | 23685571 | ||
| HCT116 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT116 cells after 72 hrs by MTT assay, IC50 = 3.24 μM. | 23685571 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells after 72 hrs by MTT assay, IC50 = 8.71 μM. | 23685571 | ||
| Vero | Cytotoxicity assay | 72 hrs | Cytotoxicity against African green monkey Vero cells after 72 hrs by MTT assay, IC50 = 9.71 μM. | 23685571 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by MTT assay, IC50 = 10.14 μM. | 23685571 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells after 72 hrs by MTT assay, IC50 = 24.96 μM. | 23685571 | ||
| PC3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human PC3 cells expressing SKP2 after 72 hrs by MTT assay, IC50 = 27.07 μM. | 23735281 | ||
| PC3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human PC3 cells expressing PCDNA after 72 hrs by MTT assay, IC50 = 31.44 μM. | 23735281 | ||
| COLO320DM | Cytotoxicity assay | 48 hrs | Cytotoxicity against human COLO320DM cells after 48 hrs by MTT assay, IC50 = 2 μM. | 23792352 | ||
| HeLa | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HeLa cells after 72 hrs by MTT assay, IC50 = 3.4 μM. | 23792763 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by MTT assay, IC50 = 3.6 μM. | 23792763 | ||
| SW480 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human SW480 cells after 72 hrs by MTT assay, IC50 = 8.1 μM. | 23792763 | ||
| MDA-MB-435S | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MDA-MB-435S cells after 72 hrs by MTT assay, IC50 = 14.5 μM. | 23792763 | ||
| LM7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human LM7 cells after 72 hrs by MTT assay, IC50 = 18.6 μM. | 23792763 | ||
| MGC803 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MGC803 cells after 72 hrs by MTT assay, IC50 = 6.92 μM. | 23792764 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by MTT assay, IC50 = 7.54 μM. | 23792764 | ||
| SMMC7721 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human SMMC7721 cells after 72 hrs by MTT assay, IC50 = 9.78 μM. | 23792764 | ||
| EC109 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human EC109 cells after 72 hrs by MTT assay, IC50 = 10.61 μM. | 23792764 | ||
| PC3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human PC3 cells after 72 hrs by MTT assay, IC50 = 24.76 μM. | 23792764 | ||
| MCF7 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MCF7 cells after 48 hrs by MTT assay, IC50 = 5 μM. | 23800391 | ||
| Vero | Antiproliferative assay | 48 hrs | Antiproliferative activity against african green monkey Vero cells after 48 hrs by MTT assay, IC50 = 8 μM. | 23800391 | ||
| WI38 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human WI38 cells after 48 hrs by MTT assay, IC50 = 10 μM. | 23800391 | ||
| HepG2 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HepG2 cells after 48 hrs by MTT assay, IC50 = 10 μM. | 23800391 | ||
| HL60 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HL60 cells after 48 hrs by MTT assay, IC50 = 22.3 μM. | 23867385 | ||
| H460 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human H460 cells after 48 hrs by MTT assay, IC50 = 26.7 μM. | 23867385 | ||
| KB | Cytotoxicity assay | 72 hrs | Cytotoxicity against human KB cells after 72 hrs by SRB assay, IC50 = 4.84 μM. | 23867603 | ||
| HeLa | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HeLa cells after 72 hrs by SRB assay, IC50 = 6.23 μM. | 23867603 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by SRB assay, IC50 = 6.53 μM. | 23867603 | ||
| OVCAR3 | Growth inhibition assay | 48 hrs | Growth inhibition of human OVCAR3 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.01 μM. | 23887055 | ||
| SR | Growth inhibition assay | 48 hrs | Growth inhibition of human SR cells after 48 hrs by sulforhodamine B assay, GI50 = 0.02 μM. | 23887055 | ||
| RPMI8226 | Growth inhibition assay | 48 hrs | Growth inhibition of human RPMI8226 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.04 μM. | 23887055 | ||
| MALME-3M | Growth inhibition assay | 48 hrs | Growth inhibition of human MALME-3M cells after 48 hrs by sulforhodamine B assay, GI50 = 0.05 μM. | 23887055 | ||
| HCC2998 | Growth inhibition assay | 48 hrs | Growth inhibition of human HCC2998 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.05 μM. | 23887055 | ||
| NCI-H460 | Growth inhibition assay | 48 hrs | Growth inhibition of human NCI-H460 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.05 μM. | 23887055 | ||
| SF539 | Growth inhibition assay | 48 hrs | Growth inhibition of human SF539 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.06 μM. | 23887055 | ||
| MCF7 | Growth inhibition assay | 48 hrs | Growth inhibition of human MCF7 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.07 μM. | 23887055 | ||
| CAKI-1 | Growth inhibition assay | 48 hrs | Growth inhibition of human CAKI-1 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.07 μM. | 23887055 | ||
| MDA-MB-435 | Growth inhibition assay | 48 hrs | Growth inhibition of human MDA-MB-435 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.07 μM. | 23887055 | ||
| HCT15 | Growth inhibition assay | 48 hrs | Growth inhibition of human HCT15 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.11 μM. | 23887055 | ||
| COLO205 | Growth inhibition assay | 48 hrs | Growth inhibition of human COLO205 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.15 μM. | 23887055 | ||
| HT-29 | Growth inhibition assay | 48 hrs | Growth inhibition of human HT-29 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.17 μM. | 23887055 | ||
| A549/ATCC | Growth inhibition assay | 48 hrs | Growth inhibition of human A549/ATCC cells after 48 hrs by sulforhodamine B assay, GI50 = 0.18 μM. | 23887055 | ||
| KM12 | Growth inhibition assay | 48 hrs | Growth inhibition of human KM12 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.21 μM. | 23887055 | ||
| HCT116 | Growth inhibition assay | 48 hrs | Growth inhibition of human HCT116 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.22 μM. | 23887055 | ||
| SF295 | Growth inhibition assay | 48 hrs | Growth inhibition of human SF295 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.22 μM. | 23887055 | ||
| LOXIMVI | Growth inhibition assay | 48 hrs | Growth inhibition of human LOXIMVI cells after 48 hrs by sulforhodamine B assay, GI50 = 0.24 μM. | 23887055 | ||
| ACHN | Growth inhibition assay | 48 hrs | Growth inhibition of human ACHN cells after 48 hrs by sulforhodamine B assay, GI50 = 0.29 μM. | 23887055 | ||
| NCI-ADR-RES | Growth inhibition assay | 48 hrs | Growth inhibition of human NCI-ADR-RES cells after 48 hrs by sulforhodamine B assay, GI50 = 0.31 μM. | 23887055 | ||
| NCI-H23 | Growth inhibition assay | 48 hrs | Growth inhibition of human NCI-H23 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.33 μM. | 23887055 | ||
| A498 | Growth inhibition assay | 48 hrs | Growth inhibition of human A498 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.35 μM. | 23887055 | ||
| MOLT4 | Growth inhibition assay | 48 hrs | Growth inhibition of human MOLT4 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.35 μM. | 23887055 | ||
| DU145 | Growth inhibition assay | 48 hrs | Growth inhibition of human DU145 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.36 μM. | 23887055 | ||
| HOP62 | Growth inhibition assay | 48 hrs | Growth inhibition of human HOP62 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.39 μM. | 23887055 | ||
| SK-MEL-5 | Growth inhibition assay | 48 hrs | Growth inhibition of human SK-MEL-5 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.46 μM. | 23887055 | ||
| SN12C | Growth inhibition assay | 48 hrs | Growth inhibition of human SN12C cells after 48 hrs by sulforhodamine B assay, GI50 = 0.49 μM. | 23887055 | ||
| UACC62 | Growth inhibition assay | 48 hrs | Growth inhibition of human UACC62 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.52 μM. | 23887055 | ||
| 786-0 | Growth inhibition assay | 48 hrs | Growth inhibition of human 786-0 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.72 μM. | 23887055 | ||
| U251 | Growth inhibition assay | 48 hrs | Growth inhibition of human U251 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.9 μM. | 23887055 | ||
| SW620 | Growth inhibition assay | 48 hrs | Growth inhibition of human SW620 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.92 μM. | 23887055 | ||
| M14 | Growth inhibition assay | 48 hrs | Growth inhibition of human M14 cells after 48 hrs by sulforhodamine B assay, GI50 = 0.98 μM. | 23887055 | ||
| SK-MEL-28 | Growth inhibition assay | 48 hrs | Growth inhibition of human SK-MEL-28 cells after 48 hrs by sulforhodamine B assay, GI50 = 1.03 μM. | 23887055 | ||
| TK10 | Growth inhibition assay | 48 hrs | Growth inhibition of human TK10 cells after 48 hrs by sulforhodamine B assay, GI50 = 1.12 μM. | 23887055 | ||
| IGROV1 | Growth inhibition assay | 48 hrs | Growth inhibition of human IGROV1 cells after 48 hrs by sulforhodamine B assay, GI50 = 1.22 μM. | 23887055 | ||
| UO31 | Growth inhibition assay | 48 hrs | Growth inhibition of human UO31 cells after 48 hrs by sulforhodamine B assay, GI50 = 1.42 μM. | 23887055 | ||
| SF268 | Growth inhibition assay | 48 hrs | Growth inhibition of human SF268 cells after 48 hrs by sulforhodamine B assay, GI50 = 1.62 μM. | 23887055 | ||
| OVCAR8 | Growth inhibition assay | 48 hrs | Growth inhibition of human OVCAR8 cells after 48 hrs by sulforhodamine B assay, GI50 = 1.74 μM. | 23887055 | ||
| HL-60(TB) | Growth inhibition assay | 48 hrs | Growth inhibition of human HL-60(TB) cells after 48 hrs by sulforhodamine B assay, GI50 = 2.3 μM. | 23887055 | ||
| PC3 | Growth inhibition assay | 48 hrs | Growth inhibition of human PC3 cells after 48 hrs by sulforhodamine B assay, GI50 = 2.36 μM. | 23887055 | ||
| RXF393 | Growth inhibition assay | 48 hrs | Growth inhibition of human RXF393 cells after 48 hrs by sulforhodamine B assay, GI50 = 2.61 μM. | 23887055 | ||
| UACC257 | Growth inhibition assay | 48 hrs | Growth inhibition of human UACC257 cells after 48 hrs by sulforhodamine B assay, GI50 = 3.55 μM. | 23887055 | ||
| K562 | Growth inhibition assay | 48 hrs | Growth inhibition of human K562 cells after 48 hrs by sulforhodamine B assay, GI50 = 3.58 μM. | 23887055 | ||
| SNB19 | Growth inhibition assay | 48 hrs | Growth inhibition of human SNB19 cells after 48 hrs by sulforhodamine B assay, GI50 = 3.81 μM. | 23887055 | ||
| OVCAR4 | Growth inhibition assay | 48 hrs | Growth inhibition of human OVCAR4 cells after 48 hrs by sulforhodamine B assay, GI50 = 4.43 μM. | 23887055 | ||
| MDA-MB-231 | Growth inhibition assay | 48 hrs | Growth inhibition of human MDA-MB-231 cells after 48 hrs by sulforhodamine B assay, GI50 = 6.6 μM. | 23887055 | ||
| NCI-H522 | Growth inhibition assay | 48 hrs | Growth inhibition of human NCI-H522 cells after 48 hrs by sulforhodamine B assay, GI50 = 7.27 μM. | 23887055 | ||
| T47D | Growth inhibition assay | 48 hrs | Growth inhibition of human T47D cells after 48 hrs by sulforhodamine B assay, GI50 = 8.12 μM. | 23887055 | ||
| Hs 578T | Growth inhibition assay | 48 hrs | Growth inhibition of human Hs 578T cells after 48 hrs by sulforhodamine B assay, GI50 = 9.77 μM. | 23887055 | ||
| CCRF-CEM | Growth inhibition assay | 48 hrs | Growth inhibition of human CCRF-CEM cells after 48 hrs by sulforhodamine B assay, GI50 = 9.79 μM. | 23887055 | ||
| BT549 | Growth inhibition assay | 48 hrs | Growth inhibition of human BT549 cells after 48 hrs by sulforhodamine B assay, GI50 = 10.6 μM. | 23887055 | ||
| OVCAR5 | Growth inhibition assay | 48 hrs | Growth inhibition of human OVCAR5 cells after 48 hrs by sulforhodamine B assay, GI50 = 10.9 μM. | 23887055 | ||
| SKOV3 | Growth inhibition assay | 48 hrs | Growth inhibition of human SKOV3 cells after 48 hrs by sulforhodamine B assay, GI50 = 21.8 μM. | 23887055 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells after 48 hrs by MTT assay, IC50 = 5.62 μM. | 23920246 | ||
| SGC7901 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human SGC7901 cells after 48 hrs by MTT assay, IC50 = 6.04 μM. | 23920246 | ||
| MDA-MB-231 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MDA-MB-231 cells after 48 hrs by MTT assay, IC50 = 13.65 μM. | 23920246 | ||
| QGY7701 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human QGY7701 cells after 48 hrs by MTT assay, IC50 = 14.3 μM. | 23920246 | ||
| SW480 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human SW480 cells after 48 hrs by MTT assay, IC50 = 15.71 μM. | 23920246 | ||
| HeLa | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HeLa cells after 48 hrs by MTT assay, IC50 = 16.52 μM. | 23920246 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells after 72 hrs by SRB assay, GI50 = 3.6 μM. | 23964677 | ||
| SKOV3 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human SKOV3 cells after 48 hrs by MTT assay, IC50 = 24.43 μM. | 23988357 | ||
| HepG2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 cells after 48 hrs by MTT assay, IC50 = 29.98 μM. | 23988357 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells after 48 hrs by MTT assay, IC50 = 34.33 μM. | 23988357 | ||
| NCI-H460 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human NCI-H460 cells after 48 hrs by MTT assay, IC50 = 44.04 μM. | 23988357 | ||
| leukemia | Cytotoxicity assay | 48 hrs | Cytotoxicity against human leukemia cells after 48 hrs by SRB assay, GI50 = 1.51 μM. | 23994328 | ||
| colon cancer | Cytotoxicity assay | 48 hrs | Cytotoxicity against human colon cancer cells after 48 hrs by SRB assay, GI50 = 8.4 μM. | 23994328 | ||
| prostate cancer | Cytotoxicity assay | 48 hrs | Cytotoxicity against human prostate cancer cells after 48 hrs by SRB assay, GI50 = 22.7 μM. | 23994328 | ||
| renal cancer | Cytotoxicity assay | 48 hrs | Cytotoxicity against human renal cancer cells after 48 hrs by SRB assay, GI50 = 45.6 μM. | 23994328 | ||
| A375-S2 | Growth inhibition assay | 48 hrs | Growth inhibition against human A375-S2 cells after 48 hrs by MTT assay, IC50 = 6.67 μM. | 23999046 | ||
| HeLa | Growth inhibition assay | 48 hrs | Growth inhibition against human HeLa cells after 48 hrs by MTT assay, IC50 = 7.34 μM. | 23999046 | ||
| HL60 | Growth inhibition assay | 48 hrs | Growth inhibition against human HL60 cells after 48 hrs by MTT assay, IC50 = 10.05 μM. | 23999046 | ||
| MCF7 | Growth inhibition assay | 48 hrs | Growth inhibition against human MCF7 cells after 48 hrs by MTT assay, IC50 = 11.5 μM. | 23999046 | ||
| K562 | Growth inhibition assay | 48 hrs | Growth inhibition against human K562 cells after 48 hrs by MTT assay, IC50 = 23.55 μM. | 23999046 | ||
| U937 | Growth inhibition assay | 48 hrs | Growth inhibition against human U937 cells after 48 hrs by MTT assay, IC50 = 23.93 μM. | 23999046 | ||
| HCT116 | Growth inhibition assay | 48 hrs | Growth inhibition against human HCT116 cells after 48 hrs by MTT assay, IC50 = 27.76 μM. | 23999046 | ||
| HT1080 | Growth inhibition assay | 48 hrs | Growth inhibition against human HT1080 cells after 48 hrs by MTT assay, IC50 = 35.62 μM. | 23999046 | ||
| HepG2 | Growth inhibition assay | 48 hrs | Growth inhibition against human HepG2 cells after 48 hrs by MTT assay, IC50 = 40.34 μM. | 23999046 | ||
| A549 | Growth inhibition assay | 48 hrs | Growth inhibition against human A549 cells after 48 hrs by MTT assay, IC50 = 44.05 μM. | 23999046 | ||
| QSG7701 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human QSG7701 cells after 48 hrs by CCK-8 assay, IC50 = 0.6 μM. | 24013415 | ||
| HepG2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 cells after 48 hrs by CCK-8 assay, IC50 = 12.48 μM. | 24013415 | ||
| SMMC7721 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human SMMC7721 cells after 48 hrs by CCK-8 assay, IC50 = 14.55 μM. | 24013415 | ||
| QGY7703 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human QGY7703 cells after 48 hrs by CCK-8 assay, IC50 = 15.66 μM. | 24013415 | ||
| Hep | Cytotoxicity assay | 48 hrs | Cytotoxicity against human Hep cells after 48 hrs by SRB assay, IC50 = 0.5 μM. | 24056146 | ||
| IMR32 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human IMR32 cells after 48 hrs by SRB assay, IC50 = 1.3 μM. | 24056146 | ||
| A549 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human A549 cells after 24 hrs by MTT assay, IC50 = 1.1 μM. | 24060486 | ||
| HeLa | Cytotoxicity assay | 24 hrs | Cytotoxicity against human HeLa cells after 24 hrs by MTT assay, IC50 = 1.1 μM. | 24060486 | ||
| MCF7 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human MCF7 cells after 24 hrs by MTT assay, IC50 = 1.2 μM. | 24060486 | ||
| DU145 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human DU145 cells after 24 hrs by MTT assay, IC50 = 1.3 μM. | 24060486 | ||
| MGC803 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MGC803 cells assessed as growth inhibition after 72 hrs by MTT assay, IC50 = 6.92 μM. | 24077182 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells assessed as growth inhibition after 72 hrs by MTT assay, IC50 = 7.54 μM. | 24077182 | ||
| SMMC7721 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human SMMC7721 cells assessed as growth inhibition after 72 hrs by MTT assay, IC50 = 9.78 μM. | 24077182 | ||
| EC109 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human EC109 cells assessed as growth inhibition after 72 hrs by MTT assay, IC50 = 10.61 μM. | 24077182 | ||
| PC3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human PC3 cells assessed as growth inhibition after 72 hrs by MTT assay, IC50 = 24.76 μM. | 24077182 | ||
| HCT8 | Antiproliferative assay | Antiproliferative activity against human HCT8 cells by MTT assay, IC50 = 6.92 μM. | 24095094 | |||
| Bel7402 | Antiproliferative assay | Antiproliferative activity against human Bel7402 cells by MTT assay, IC50 = 8.3 μM. | 24095094 | |||
| A549 | Antiproliferative assay | Antiproliferative activity against human A549 cells by MTT assay, IC50 = 8.92 μM. | 24095094 | |||
| SKOV3 | Cytotoxicity assay | 3 days | Cytotoxicity against human SKOV3 cells after 3 days by MTT assay, IC50 = 24.43 μM. | 24095745 | ||
| HepG2 | Cytotoxicity assay | 3 days | Cytotoxicity against human HepG2 cells after 3 days by MTT assay, IC50 = 29.98 μM. | 24095745 | ||
| A549 | Cytotoxicity assay | 3 days | Cytotoxicity against human A549 cells after 3 days by MTT assay, IC50 = 34.33 μM. | 24095745 | ||
| NCI-H460 | Cytotoxicity assay | 3 days | Cytotoxicity against human NCI-H460 cells after 3 days by MTT assay, IC50 = 44.04 μM. | 24095745 | ||
| MGC803 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MGC803 cells after 48 hrs by MTT assay, IC50 = 3.68 μM. | 24119869 | ||
| HepG2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 cells after 48 hrs by MTT assay, IC50 = 4.35 μM. | 24119869 | ||
| Bcap37 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human Bcap37 cells after 48 hrs by MTT assay, IC50 = 5.58 μM. | 24119869 | ||
| SGC7901 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human SGC7901 cells after 48 hrs by MTT assay, IC50 = 7.37 μM. | 24119869 | ||
| DU145 | Cytotoxicity assay | Cytotoxic activity against human DU145 cells, IC50 = 3.4 μM. | 24195466 | |||
| NCI-H460 | Cytotoxicity assay | Cytotoxic activity against human NCI-H460 cells, IC50 = 8.5 μM. | 24195466 | |||
| HCT116 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT116 cells after 72 hrs by MTT assay, IC50 = 8.32 μM. | 24211021 | ||
| MGC803 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MGC803 cells after 72 hrs by MTT assay, IC50 = 15.32 μM. | 24211021 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells after 72 hrs by MTT assay, IC50 = 19.45 μM. | 24211021 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells after 72 hrs by MTT assay, IC50 = 20.13 μM. | 24211021 | ||
| NCI-H460 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human NCI-H460 cells after 72 hrs by MTT assay, IC50 = 23.32 μM. | 24211021 | ||
| SW1116 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human SW1116 cells after 48 hrs by MTT assay, IC50 = 5.26 μM. | 24286761 | ||
| HepG2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 cells after 48 hrs by MTT assay, IC50 = 7.43 μM. | 24286761 | ||
| BGC823 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human BGC823 cells after 48 hrs by MTT assay, IC50 = 7.48 μM. | 24286761 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MCF7 cells after 48 hrs by MTT assay, IC50 = 9.79 μM. | 24286761 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MCF7 cells after 48 hrs by MTT assay, IC50 = 3.5 μM. | 24332497 | ||
| B16 | Cytotoxicity assay | 48 hrs | Cytotoxicity against mouse B16 cells after 48 hrs by MTT assay, IC50 = 4.2 μM. | 24332497 | ||
| ME180 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human ME180 cells after 48 hrs by MTT assay, IC50 = 4.9 μM. | 24332497 | ||
| A549 | Cytotoxicity assay | 4 days | Cytotoxicity against human A549 cells after 4 days by MTT assay, IC50 = 3.1 μM. | 24359303 | ||
| Bel7402 | Cytotoxicity assay | 4 days | Cytotoxicity against human Bel7402 cells after 4 days by MTT assay, IC50 = 5.5 μM. | 24359303 | ||
| KB | Cytotoxicity assay | 4 days | Cytotoxicity against human KB cells after 4 days by MTT assay, IC50 = 8.1 μM. | 24359303 | ||
| HCT116 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HCT116 cells after 48 hrs by MTT assay, IC50 = 5.78 μM. | 24378217 | ||
| HepG2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 cells after 48 hrs by MTT assay, IC50 = 29.98 μM. | 24378217 | ||
| HeLa | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HeLa cells after 48 hrs by MTT assay, IC50 = 35.34 μM. | 24378217 | ||
| CNE | Cytotoxicity assay | 48 hrs | Cytotoxicity against human CNE cells after 48 hrs by MTT assay, IC50 = 40.21 μM. | 24378217 | ||
| SPCA2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human SPCA2 cells after 48 hrs by MTT assay, IC50 = 42.2 μM. | 24378217 | ||
| colon cancer | Cytotoxicity assay | 10 uM | 48 hrs | Cytotoxicity against human colon cancer cells at 10 uM after 48 hrs, GI50 = 8.4 μM. | 24469112 | |
| leukemia | Cytotoxicity assay | 10 uM | 48 hrs | Cytotoxicity against human leukemia cells at 10 uM after 48 hrs, GI50 = 15.1 μM. | 24469112 | |
| prostate cancer | Cytotoxicity assay | 10 uM | 48 hrs | Cytotoxicity against human prostate cancer cells at 10 uM after 48 hrs, GI50 = 22.7 μM. | 24469112 | |
| renal cancer | Cytotoxicity assay | 10 uM | 48 hrs | Cytotoxicity against human renal cancer cells at 10 uM after 48 hrs, GI50 = 45.6 μM. | 24469112 | |
| EAC | Cytotoxicity assay | 48 hrs | Cytotoxicity against mouse EAC cells after 48 hrs by MTT assay, IC50 = 14.3 μM. | 24534537 | ||
| DLA | Cytotoxicity assay | 48 hrs | Cytotoxicity against mouse DLA cells after 48 hrs by trypan blue exclusion assay, IC50 = 14.3 μM. | 24534537 | ||
| DLA | Cytotoxicity assay | 48 hrs | Cytotoxicity against mouse DLA cells after 48 hrs by MTT assay, IC50 = 15.7 μM. | 24534537 | ||
| DLA | Cytotoxicity assay | 48 hrs | Cytotoxicity against mouse DLA cells after 48 hrs by LDH release assay, IC50 = 15.8 μM. | 24534537 | ||
| EAC | Cytotoxicity assay | 48 hrs | Cytotoxicity against mouse EAC cells after 48 hrs by LDH release assay, IC50 = 16.3 μM. | 24534537 | ||
| EAC | Cytotoxicity assay | 48 hrs | Cytotoxicity against mouse EAC cells after 48 hrs by trypan blue exclusion assay, IC50 = 16.4 μM. | 24534537 | ||
| HeLa | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HeLa cells after 48 hrs by MTT assay, IC50 = 2.3 μM. | 24547794 | ||
| HepG2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 cells after 48 hrs by MTT assay, IC50 = 7.6 μM. | 24547794 | ||
| HCT116 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HCT116 cells after 48 hrs by MTT assay, IC50 = 24.43 μM. | 24565905 | ||
| SKOV3 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human SKOV3 cells after 48 hrs by MTT assay, IC50 = 24.43 μM. | 24565905 | ||
| Bel7404 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human Bel7404 cells after 48 hrs by MTT assay, IC50 = 26.8 μM. | 24565905 | ||
| HepG2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 cells after 48 hrs by MTT assay, IC50 = 26.98 μM. | 24565905 | ||
| NCI-H460 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human NCI-H460 cells after 48 hrs by MTT assay, IC50 = 36.04 μM. | 24565905 | ||
| H3347 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human H3347 cells assessed as growth inhibition after 72 hrs by Alamar Blue assay, IC50 = 11.64 μM. | 24583355 | ||
| HT-29 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HT-29 cells assessed as growth inhibition after 72 hrs by Alamar Blue assay, IC50 = 24.87 μM. | 24583355 | ||
| DLD1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human DLD1 cells assessed as growth inhibition after 72 hrs by Alamar Blue assay, IC50 = 29.14 μM. | 24583355 | ||
| L1210 | Cytotoxicity assay | 48 hrs | Cytotoxicity against mouse L1210 cells assessed as reduction in cell number after 48 hrs by cell counting assay, IC50 = 0.33 μM. | 24631358 | ||
| HeLa | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HeLa cells assessed as reduction in cell number after 72 hrs by cell counting assay, IC50 = 0.54 μM. | 24631358 | ||
| CCRF-CEM | Cytotoxicity assay | 72 hrs | Cytotoxicity against human CCRF-CEM cells assessed as reduction in cell number after 72 hrs by cell counting assay, IC50 = 18 μM. | 24631358 | ||
| HeLa | Cytotoxicity assay | 24 hrs | Cytotoxicity against human HeLa cells assessed as growth inhibition after 24 hrs by MTT assay, IC50 = 1.8 μM. | 24641975 | ||
| MCF7 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human MCF7 cells assessed as growth inhibition after 24 hrs by MTT assay, IC50 = 1.8 μM. | 24641975 | ||
| A549 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human A549 cells assessed as growth inhibition after 24 hrs by MTT assay, IC50 = 1.9 μM. | 24641975 | ||
| DU145 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human DU145 cells assessed as growth inhibition after 24 hrs by MTT assay, IC50 = 1.9 μM. | 24641975 | ||
| COLO205 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human COLO205 cells assessed as inhibition of tumor growth after 24 hrs, IC50 = 3.39 μM. | 24657568 | ||
| RPMI8226 | Antileukemic assay | 24 hrs | Antileukemic activity against human RPMI8226 cells assessed as inhibition of tumor growth after 24 hrs, IC50 = 3.55 μM. | 24657568 | ||
| HCC2998 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human HCC2998 cells assessed as inhibition of tumor growth after 24 hrs, IC50 = 5.75 μM. | 24657568 | ||
| HCT15 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human HCT15 cells assessed as inhibition of tumor growth after 24 hrs, IC50 = 6.61 μM. | 24657568 | ||
| KM12 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human KM12 cells assessed as inhibition of tumor growth after 24 hrs, IC50 = 7.94 μM. | 24657568 | ||
| SW620 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human SW620 cells assessed as inhibition of tumor growth after 24 hrs, IC50 = 18.6 μM. | 24657568 | ||
| NCI60 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human NCI60 cells assessed as inhibition of tumor growth after 24 hrs, IC50 = 29.5 μM. | 24657568 | ||
| E0771 | Cytotoxicity assay | 3 days | Cytotoxicity against mouse E0771 cells after 3 days by SRB assay, IC50 = 0.28 μM. | 24704691 | ||
| HT-29 | Cytotoxicity assay | 3 days | Cytotoxicity against human HT-29 cells after 3 days by SRB assay, IC50 = 2.58 μM. | 24704691 | ||
| MCF7 | Cytotoxicity assay | 3 days | Cytotoxicity against human MCF7 cells after 3 days by SRB assay, IC50 = 2.86 μM. | 24704691 | ||
| SW480 | Cytotoxicity assay | 3 days | Cytotoxicity against human SW480 cells after 3 days by SRB assay, IC50 = 3.69 μM. | 24704691 | ||
| RKO | Cytotoxicity assay | 3 days | Cytotoxicity against human RKO cells after 3 days by SRB assay, IC50 = 4.39 μM. | 24704691 | ||
| MCF10A | Cytotoxicity assay | 3 days | Cytotoxicity against human MCF10A cells after 3 days by SRB assay, IC50 = 8.06 μM. | 24704691 | ||
| CCD-18Co | Cytotoxicity assay | 3 days | Cytotoxicity against human CCD-18Co cells after 3 days by SRB assay, IC50 = 9.26 μM. | 24704691 | ||
| T47D | Cytotoxicity assay | 3 days | Cytotoxicity against human T47D cells after 3 days by SRB assay, IC50 = 10.56 μM. | 24704691 | ||
| MDA-MB-231 | Cytotoxicity assay | 3 days | Cytotoxicity against human MDA-MB-231 cells after 3 days by SRB assay, IC50 = 18.12 μM. | 24704691 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells assessed as growth inhibition measured at 48 hrs by SRB assay, IC50 = 0.98 μM. | 24727462 | ||
| HeLa | Cytotoxicity assay | 2 days | Cytotoxicity against human HeLa cells after 2 days by MTT assay, IC50 = 7.34 μM. | 24767839 | ||
| MCF7 | Cytotoxicity assay | 2 days | Cytotoxicity against human MCF7 cells after 2 days by MTT assay, IC50 = 11.5 μM. | 24767839 | ||
| HT-29 | Cytotoxicity assay | 2 days | Cytotoxicity against human HT-29 cells after 2 days by MTT assay, IC50 = 17.2 μM. | 24767839 | ||
| A549 | Cytotoxicity assay | 2 days | Cytotoxicity against human A549 cells after 2 days by MTT assay, IC50 = 44.05 μM. | 24767839 | ||
| A549 | Growth inhibition assay | 48 hrs | Growth inhibition of human A549 cells after 48 hrs by SRB assay, GI50 = 4.55 μM. | 24797798 | ||
| PC3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human PC3 cells after 72 hrs by MTT assay, IC50 = 4.68 μM. | 24798098 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by MTT assay, IC50 = 7.76 μM. | 24798098 | ||
| MGC803 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MGC803 cells after 72 hrs by MTT assay, IC50 = 8.13 μM. | 24798098 | ||
| EC109 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human EC109 cells after 72 hrs by MTT assay, IC50 = 24.14 μM. | 24798098 | ||
| MCF7 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MCF7 cells after 72 hrs by MTT assay, IC50 = 7.08 μM. | 24819956 | ||
| HCT116 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT116 cells after 72 hrs by MTT assay, IC50 = 10.54 μM. | 24819956 | ||
| Bel7402 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human Bel7402 cells after 72 hrs by MTT assay, IC50 = 11.12 μM. | 24819956 | ||
| A549 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human A549 cells after 24 hrs by MTT assay, IC50 = 1.3 μM. | 24835633 | ||
| HeLa | Cytotoxicity assay | 24 hrs | Cytotoxicity against human HeLa cells after 24 hrs by MTT assay, IC50 = 1.3 μM. | 24835633 | ||
| MCF7 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human MCF7 cells after 24 hrs by MTT assay, IC50 = 1.4 μM. | 24835633 | ||
| DU145 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human DU145 cells after 24 hrs by MTT assay, IC50 = 1.5 μM. | 24835633 | ||
| MCF7 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MCF7 cells after 48 hrs by MTT assay, IC50 = 3.52 μM. | 24835817 | ||
| EC9706 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human EC9706 cells after 48 hrs by MTT assay, IC50 = 9.45 μM. | 24835817 | ||
| MGC803 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MGC803 cells after 48 hrs by MTT assay, IC50 = 11.34 μM. | 24835817 | ||
| L1210 | Cytostatic activity assay | 48 hrs | Cytostatic activity against mouse L1210 cells assessed as inhibition of proliferation after 48 hrs by Coulter cell counting method, IC50 = 0.33 μM. | 24906510 | ||
| HeLa | Cytostatic activity assay | 72 hrs | Cytostatic activity against human HeLa cells assessed as inhibition of proliferation after 72 hrs by Coulter cell counting method, IC50 = 0.54 μM. | 24906510 | ||
| CEM | Cytostatic activity assay | 72 hrs | Cytostatic activity against human CEM cells assessed as inhibition of proliferation after 72 hrs by Coulter cell counting method, IC50 = 18 μM. | 24906510 | ||
| PC3 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human PC3 cells after 48 hrs by sulforhodamine B assay, IC50 = 2.2 μM. | 24910974 | ||
| HCT15 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HCT15 cells after 48 hrs by sulforhodamine B assay, IC50 = 4.5 μM. | 24910974 | ||
| HeLa | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HeLa cells after 48 hrs by sulforhodamine B assay, IC50 = 4.9 μM. | 24910974 | ||
| WM266.4 | Antiproliferative assay | 24 hrs | Antiproliferative activity against human WM266.4 cells after 24 hrs by MTT assay, GI50 = 28.76 μM. | 24934992 | ||
| HeLa | Antiproliferative assay | 24 hrs | Antiproliferative activity against human HeLa cells after 24 hrs by MTT assay, GI50 = 30.21 μM. | 24934992 | ||
| MDA-MB-231 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MDA-MB-231 cells after 48 hrs by MTT assay, IC50 = 2.45 μM. | 24941129 | ||
| HeLa | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HeLa cells after 48 hrs by MTT assay, IC50 = 2.78 μM. | 24941129 | ||
| KCL22 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human KCL22 cells after 48 hrs by MTT assay, IC50 = 3.52 μM. | 24941129 | ||
| KB | Cytotoxicity assay | 48 hrs | Cytotoxicity against human KB cells after 48 hrs by MTT assay, IC50 = 1.23 μM. | 24953029 | ||
| HCT116 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HCT116 cells after 48 hrs by MTT assay, IC50 = 10.05 μM. | 24953029 | ||
| HeLa | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HeLa cells after 48 hrs by MTT assay, IC50 = 23.33 μM. | 24953029 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells after 48 hrs by MTT assay, IC50 = 34.34 μM. | 24953029 | ||
| HepG2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 cells after 48 hrs by MTT assay, IC50 = 38.34 μM. | 24953029 | ||
| CNE | Cytotoxicity assay | 48 hrs | Cytotoxicity against human CNE cells after 48 hrs by MTT assay, IC50 = 45.1 μM. | 24953029 | ||
| NCI-H460 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human NCI-H460 cells after 48 hrs by MTT assay, IC50 = 45.14 μM. | 24953029 | ||
| MGC803 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MGC803 cells after 48 hrs by MTT assay, IC50 = 46.93 μM. | 24953029 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells after 48 hrs by sulforhodamine B assay, IC50 = 4.21 μM. | 24956552 | ||
| PC3 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human PC3 cells after 48 hrs by sulforhodamine B assay, IC50 = 12 μM. | 24956552 | ||
| A549 | Cytotoxicity assay | 24 to 48 hrs | Cytotoxicity against human A549 cells assessed as growth inhibition after 24 to 48 hrs by sulforhodamine B assay, IC50 = 1.65 μM. | 24996138 | ||
| MGC803 | Anticancer assay | 72 hrs | Anticancer activity against human MGC803 cells after 72 hrs by MTT assay, IC50 = 3.45 μM. | 25001482 | ||
| MCF7 | Anticancer assay | 72 hrs | Anticancer activity against human MCF7 cells after 72 hrs by MTT assay, IC50 = 7.12 μM. | 25001482 | ||
| EC9706 | Anticancer assay | 72 hrs | Anticancer activity against human EC9706 cells after 72 hrs by MTT assay, IC50 = 8.07 μM. | 25001482 | ||
| SSMC-7721 | Anticancer assay | 72 hrs | Anticancer activity against human SSMC-7721 cells after 72 hrs by MTT assay, IC50 = 15.08 μM. | 25001482 | ||
| MDA-MB-231 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MDA-MB-231 cells after 48 hrs by MTT assay, IC50 = 24.6 μM. | 25016234 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells after 48 hrs by MTT assay, IC50 = 35.41 μM. | 25016234 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MCF7 cells after 48 hrs by MTT assay, IC50 = 39.88 μM. | 25016234 | ||
| HepG2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 cells after 48 hrs by MTT assay, IC50 = 46.83 μM. | 25016234 | ||
| HCT116 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT116 cells after 72 hrs by MTS assay, IC50 = 0.5 μM. | 25066282 | ||
| SW620 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human SW620 cells after 72 hrs by MTS assay, IC50 = 5.3 μM. | 25066282 | ||
| MCF7 | Anticancer assay | 72 hrs | Anticancer activity against human MCF7 cells assessed as inhibition of cell proliferation after 72 hrs by MTT assay, IC50 = 4.6 μM. | 25086915 | ||
| EC9706 | Anticancer assay | 72 hrs | Anticancer activity against human EC9706 cells assessed as inhibition of cell proliferation after 72 hrs by MTT assay, IC50 = 4.7 μM. | 25086915 | ||
| MGC803 | Anticancer assay | 72 hrs | Anticancer activity against human MGC803 cells assessed as inhibition of cell proliferation after 72 hrs by MTT assay, IC50 = 10 μM. | 25086915 | ||
| HeLa | Anticancer assay | 72 hrs | Anticancer activity against human HeLa cells assessed as inhibition of cell proliferation after 72 hrs by MTT assay, IC50 = 12 μM. | 25086915 | ||
| A549 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human A549 cells assessed as inhibition of cell viability after 48 hrs by MTT assay, IC50 = 7.62 μM. | 25113877 | ||
| MGC803 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MGC803 cells assessed as inhibition of cell viability after 48 hrs by MTT assay, IC50 = 7.76 μM. | 25113877 | ||
| CNE | Antiproliferative assay | 48 hrs | Antiproliferative activity against human CNE cells assessed as inhibition of cell viability after 48 hrs by MTT assay, IC50 = 15.07 μM. | 25113877 | ||
| Bel7402 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human Bel7402 cells assessed as inhibition of cell viability after 48 hrs by MTT assay, IC50 = 19.84 μM. | 25113877 | ||
| HepG2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 cells after 48 hrs by MTT assay, IC50 = 29.98 μM. | 25151580 | ||
| HeLa | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HeLa cells after 48 hrs by MTT assay, IC50 = 35.34 μM. | 25151580 | ||
| SMMC-7404 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human SMMC-7404 cells after 48 hrs by MTT assay, IC50 = 40.21 μM. | 25151580 | ||
| NCI-H460 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human NCI-H460 cells after 48 hrs by MTT assay, IC50 = 44.04 μM. | 25151580 | ||
| MGC803 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MGC803 cells after 48 hrs by MTT assay, IC50 = 46.93 μM. | 25151580 | ||
| B16 | Antiproliferative assay | 48 hrs | Antiproliferative activity against mouse B16 cells after 48 hrs by MTT assay, IC50 = 17.85 μM. | 25164762 | ||
| HL60 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HL60 cells after 48 hrs by MTT assay, IC50 = 27.01 μM. | 25164762 | ||
| Hep | Cytotoxicity assay | 48 hrs | Cytotoxicity against human Hep cells after 48 hrs by SRB assay, IC50 = 0.5 μM. | 25176329 | ||
| IMR32 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human IMR32 cells after 48 hrs by SRB assay, IC50 = 1.3 μM. | 25176329 | ||
| B16F10 | Cytotoxicity assay | 72 hrs | Cytotoxicity against mouse B16F10 cells assessed as inhibition of proliferation after 72 hrs by MTT assay, IC50 = 0.87 μM. | 25180925 | ||
| MGC803 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MGC803 cells assessed as inhibition of proliferation after 72 hrs by MTT assay, IC50 = 7.69 μM. | 25180925 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells assessed as inhibition of proliferation after 72 hrs by MTT assay, IC50 = 8.93 μM. | 25180925 | ||
| EC109 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human EC109 cells assessed as inhibition of proliferation after 72 hrs by MTT assay, IC50 = 10.81 μM. | 25180925 | ||
| HEK293 | Antiproliferative assay | 24 hrs | Antiproliferative activity against HEK293 cells after 24 hrs by MTT assay, IC50 = 48.7 μM. | 25198997 | ||
| Bel7402 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human Bel7402 cells assessed as growth inhibition rate after 96 hrs by MTT assay, IC50 = 2.23 μM. | 25203783 | ||
| HCT8 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human HCT8 cells assessed as growth inhibition rate after 96 hrs by MTT assay, IC50 = 3.54 μM. | 25203783 | ||
| C33A | Cytotoxicity assay | 96 hrs | Cytotoxicity against human C33A cells assessed as growth inhibition rate after 96 hrs by MTT assay, IC50 = 4.61 μM. | 25203783 | ||
| A549 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human A549 cells assessed as growth inhibition rate after 96 hrs by MTT assay, IC50 = 6.69 μM. | 25203783 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells after 48 hrs by SRB assay, IC50 = 0.01 μM. | 25259515 | ||
| THP1 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human THP1 cells after 48 hrs by SRB assay, IC50 = 0.04 μM. | 25259515 | ||
| EAC | Cytotoxicity assay | 48 hrs | Cytotoxicity against mouse EAC cells after 48 hrs by trypan blue dye exclusion method, IC50 = 14.6 μM. | 25261825 | ||
| DLA | Cytotoxicity assay | 48 hrs | Cytotoxicity against mouse DLA cells after 48 hrs by trypan blue dye exclusion method, IC50 = 15.3 μM. | 25261825 | ||
| EAC | Cytotoxicity assay | 48 hrs | Cytotoxicity against mouse EAC cells after 48 hrs by LDH release assay, IC50 = 15.4 μM. | 25261825 | ||
| DLA | Cytotoxicity assay | 48 hrs | Cytotoxicity against mouse DLA cells after 48 hrs by MTT method, IC50 = 15.6 μM. | 25261825 | ||
| EAC | Cytotoxicity assay | 48 hrs | Cytotoxicity against mouse EAC cells after 48 hrs by MTT method, IC50 = 16.3 μM. | 25261825 | ||
| DLA | Cytotoxicity assay | 48 hrs | Cytotoxicity against mouse DLA cells after 48 hrs by LDH release assay, IC50 = 16.8 μM. | 25261825 | ||
| L1210 | Cytostatic activity assay | 48 hrs | Cytostatic activity against mouse L1210 cells after 48 hrs by Coulter counting, IC50 = 0.33 μM. | 25435471 | ||
| HeLa | Cytostatic activity assay | 72 hrs | Cytostatic activity against human HeLa cells after 72 hrs by Coulter counting, IC50 = 2.6 μM. | 25435471 | ||
| CEM | Cytostatic activity assay | 72 hrs | Cytostatic activity against human CEM cells after 72 hrs by Coulter counting, IC50 = 18 μM. | 25435471 | ||
| SMMC7721 | Antiproliferative assay | Antiproliferative activity against human SMMC7721 cells assessed as inhibition of cell proliferation by CCK8 assay, IC50 = 5.62 μM. | 25442303 | |||
| A549 | Antiproliferative assay | Antiproliferative activity against human A549 cells assessed as inhibition of cell proliferation by CCK8 assay, IC50 = 8.13 μM. | 25442303 | |||
| MCF7 | Antiproliferative assay | Antiproliferative activity against human MCF7 cells assessed as inhibition of cell proliferation by CCK8 assay, IC50 = 14.26 μM. | 25442303 | |||
| LoVo | Antiproliferative assay | 48 hrs | Antiproliferative activity against human LoVo cells assessed as cell-growth inhibition after 48 hrs by MTT assay, IC50 = 12.7 μM. | 25453794 | ||
| MCF7 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MCF7 cells assessed as cell-growth inhibition after 48 hrs by MTT assay, IC50 = 16.3 μM. | 25453794 | ||
| HeLa | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HeLa cells assessed as cell-growth inhibition after 48 hrs by MTT assay, IC50 = 18.1 μM. | 25453794 | ||
| BGC823 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human BGC823 cells assessed as cell-growth inhibition after 48 hrs by MTT assay, IC50 = 35.6 μM. | 25453794 | ||
| HCT116 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HCT116 cells assessed as cell-growth inhibition after 48 hrs by MTT assay, IC50 = 36.2 μM. | 25453794 | ||
| U87 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human U87 cells assessed as cell-growth inhibition after 48 hrs by MTT assay, IC50 = 38.2 μM. | 25453794 | ||
| MGC803 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MGC803 cells after 48 hrs by MTT assay, IC50 = 30.45 μM. | 25462253 | ||
| NCI-H460 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human NCI-H460 cells after 48 hrs by MTT assay, IC50 = 36.04 μM. | 25462253 | ||
| HeLa | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HeLa cells after 48 hrs by MTT assay, IC50 = 36.53 μM. | 25462253 | ||
| MCF7 | Anticancer assay | 48 hrs | Anticancer activity against human MCF7 cells after 48 hrs by MTT assay, IC50 = 26.9 μM. | 25462264 | ||
| GES-1 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human GES-1 cells after 48 hrs by MTT assay, IC50 = 30.9 μM. | 25462264 | ||
| K562 | Anticancer assay | 48 hrs | Anticancer activity against human K562 cells after 48 hrs by MTT assay, IC50 = 31.6 μM. | 25462264 | ||
| HCT116 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT116 cells after 72 hrs by SRB assay, IC50 = 6 μM. | 25462277 | ||
| T47D | Cytotoxicity assay | 72 hrs | Cytotoxicity against human T47D cells after 72 hrs by SRB assay, IC50 = 7.9 μM. | 25462277 | ||
| HuH7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HuH7 cells after 72 hrs by SRB assay, IC50 = 30.7 μM. | 25462277 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MCF7 cells assessed as cell growth inhibition after 48 hrs by WST-1 assay, IC50 = 34.48 μM. | 25466196 | ||
| SKBR3 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human SKBR3 cells overexpressing HER2 assessed as cell growth inhibition after 48 hrs by WST-1 assay, IC50 = 38.88 μM. | 25466196 | ||
| B16 | Cytotoxicity assay | 48 hrs | Cytotoxicity against mouse B16 cells assessed as cell survival after 48 hrs by MTT assay, IC50 = 7.64 μM. | 25506718 | ||
| MKN28 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MKN28 cells assessed as cell survival after 48 hrs by MTT assay, IC50 = 9.67 μM. | 25506718 | ||
| HeLa | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HeLa cells assessed as cell survival after 48 hrs by MTT assay, IC50 = 13.45 μM. | 25506718 | ||
| HeLa | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HeLa cells after 72 hrs by MTT assay, IC50 = 9.09 μM. | 25529742 | ||
| SF763 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SF763 cells after 72 hrs by MTT assay, IC50 = 9.34 μM. | 25529742 | ||
| HL60 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HL60 cells after 72 hrs by MTT assay, IC50 = 10.45 μM. | 25529742 | ||
| B16 | Antiproliferative assay | 72 hrs | Antiproliferative activity against mouse B16 cells after 72 hrs by MTT assay, IC50 = 10.52 μM. | 25529742 | ||
| Bel7402 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human Bel7402 cells after 72 hrs by MTT assay, IC50 = 11.78 μM. | 25529742 | ||
| MGC803 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MGC803 cells after 48 hrs by MTT assay, IC50 = 3.71 μM. | 25554922 | ||
| Bcap37 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human Bcap37 cells after 48 hrs by MTT assay, IC50 = 5.18 μM. | 25554922 | ||
| SGC7901 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human SGC7901 cells after 48 hrs by MTT assay, IC50 = 6.56 μM. | 25554922 | ||
| BALB/3T3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against mouse BALB/3T3 cells assessed as growth inhibition after 72 hrs by SRB method, IC50 = 3.23 μM. | 25644674 | ||
| LoVo | Antiproliferative assay | 72 hrs | Antiproliferative activity against human LoVo cells assessed as growth inhibition after 72 hrs by SRB method, IC50 = 4.69 μM. | 25644674 | ||
| doxorubicin-resistant LoVo/DX | Antiproliferative assay | 72 hrs | Antiproliferative activity against human doxorubicin-resistant LoVo/DX cells assessed as growth inhibition after 72 hrs by SRB method, IC50 = 6 μM. | 25644674 | ||
| SW707 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SW707 cells assessed as growth inhibition after 72 hrs by SRB method, IC50 = 20.46 μM. | 25644674 | ||
| vincristine-resistant HL60/Vinc | Antiproliferative assay | 72 hrs | Antiproliferative activity against human vincristine-resistant HL60/Vinc cells assessed as growth inhibition after 72 hrs by MTT assay, IC50 = 21.15 μM. | 25644674 | ||
| HL60 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HL60 cells assessed as growth inhibition after 72 hrs by MTT assay, IC50 = 22.54 μM. | 25644674 | ||
| HT-29 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HT-29 cells assessed as growth inhibition after 72 hrs by SRB method, IC50 = 25.08 μM. | 25644674 | ||
| LS180 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human LS180 cells assessed as growth inhibition after 72 hrs by SRB method, IC50 = 38.92 μM. | 25644674 | ||
| B16F10 | Anticancer assay | 72 hrs | Anticancer activity against mouse B16F10 cells assessed as inhibition of proliferation after 72 hrs by MTT assay, IC50 = 1.43 μM. | 25655718 | ||
| MGC803 | Anticancer assay | 72 hrs | Anticancer activity against human MGC803 cells assessed as inhibition of proliferation after 72 hrs by MTT assay, IC50 = 8.43 μM. | 25655718 | ||
| MCF7 | Anticancer assay | 72 hrs | Anticancer activity against human MCF7 cells assessed as inhibition of proliferation after 72 hrs by MTT assay, IC50 = 9.12 μM. | 25655718 | ||
| EC109 | Anticancer assay | 72 hrs | Anticancer activity against human EC109 cells assessed as inhibition of proliferation after 72 hrs by MTT assay, IC50 = 11.61 μM. | 25655718 | ||
| HCT8 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT8 cells assessed as inhibition of cell proliferation after 72 hrs by MTT assay, IC50 = 0.91 μM. | 25675144 | ||
| HCT8 | Antiproliferative assay | 72 hrs | Antiproliferative activity against 5-FU resistant human HCT8 cells assessed as inhibition of cell proliferation after 72 hrs by MTT assay, IC50 = 36.638 μM. | 25675144 | ||
| MGC803 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MGC803 cells after 72 hrs by MTT assay, IC50 = 10.72 μM. | 25707012 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by MTT assay, IC50 = 12.24 μM. | 25707012 | ||
| EC9706 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human EC9706 cells after 72 hrs by MTT assay, IC50 = 15.67 μM. | 25707012 | ||
| SMMC7721 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human SMMC7721 cells after 72 hrs by MTT assay, IC50 = 20.4 μM. | 25707012 | ||
| SW1573 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human SW1573 cells after 48 hrs by sulforhodamine B assay, GI50 = 4.3 μM. | 25752525 | ||
| HBL100 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HBL100 cells after 48 hrs by sulforhodamine B assay, GI50 = 5.5 μM. | 25752525 | ||
| HeLa | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HeLa cells after 48 hrs by sulforhodamine B assay, GI50 = 15 μM. | 25752525 | ||
| T47D | Antiproliferative assay | 48 hrs | Antiproliferative activity against human T47D cells after 48 hrs by sulforhodamine B assay, GI50 = 47 μM. | 25752525 | ||
| WiDr | Antiproliferative assay | 48 hrs | Antiproliferative activity against human WiDr cells after 48 hrs by sulforhodamine B assay, GI50 = 49 μM. | 25752525 | ||
| MGC803 | Anticancer assay | 48 hrs | Anticancer activity against human MGC803 cells assessed as cell survival after 48 hrs by MTT assay, IC50 = 3.58 μM. | 25812966 | ||
| SMMC7721 | Anticancer assay | 48 hrs | Anticancer activity against human SMMC7721 cells assessed as cell survival after 48 hrs by MTT assay, IC50 = 4.87 μM. | 25812966 | ||
| HepG2 | Anticancer assay | 48 hrs | Anticancer activity against human HepG2 cells assessed as cell survival after 48 hrs by MTT assay, IC50 = 5.86 μM. | 25812966 | ||
| SGC7901 | Anticancer assay | 48 hrs | Anticancer activity against human SGC7901 cells assessed as cell survival after 48 hrs by MTT assay, IC50 = 6.12 μM. | 25812966 | ||
| SKOV3 | Cytotoxicity assay | 2 days | Cytotoxicity against human SKOV3 cells after 2 days by MTT assay, IC50 = 27.34 μM. | 25841196 | ||
| HepG2 | Cytotoxicity assay | 2 days | Cytotoxicity against human HepG2 cells after 2 days by MTT assay, IC50 = 31.98 μM. | 25841196 | ||
| A549 | Cytotoxicity assay | 2 days | Cytotoxicity against human A549 cells after 2 days by MTT assay, IC50 = 38.23 μM. | 25841196 | ||
| T24 | Cytotoxicity assay | 2 days | Cytotoxicity against human T24 cells after 2 days by MTT assay, IC50 = 40.57 μM. | 25841196 | ||
| SPCA2 | Cytotoxicity assay | 2 days | Cytotoxicity against human SPCA2 cells after 2 days by MTT assay, IC50 = 44.1 μM. | 25841196 | ||
| NCI-H460 | Cytotoxicity assay | 2 days | Cytotoxicity against human NCI-H460 cells after 2 days by MTT assay, IC50 = 46.15 μM. | 25841196 | ||
| MGC803 | Cytotoxicity assay | 2 days | Cytotoxicity against human MGC803 cells after 2 days by MTT assay, IC50 = 47.39 μM. | 25841196 | ||
| HCT116 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HCT116 cells assessed as cell viability after 48 hrs by MTT assay, IC50 = 29.58 μM. | 25841199 | ||
| HepG2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 cells assessed as cell viability after 48 hrs by MTT assay, IC50 = 30.79 μM. | 25841199 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells assessed as cell viability after 48 hrs by MTT assay, IC50 = 36.34 μM. | 25841199 | ||
| T24 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human T24 cells assessed as cell viability after 48 hrs by MTT assay, IC50 = 37.56 μM. | 25841199 | ||
| MGC803 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MGC803 cells assessed as cell viability after 48 hrs by MTT assay, IC50 = 40.94 μM. | 25841199 | ||
| MDA-MB-231 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MDA-MB-231 cells after 48 hrs by MTT assay, IC50 = 5.82 μM. | 25870131 | ||
| DU145 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human DU145 cells after 48 hrs by MTT assay, IC50 = 6.37 μM. | 25870131 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells after 48 hrs by MTT assay, IC50 = 7.51 μM. | 25870131 | ||
| A549 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A549 cells after 72 hrs by WST8 assay, IC50 = 2.8 μM. | 25919052 | ||
| PANC1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human PANC1 cells after 72 hrs by WST8 assay, IC50 = 3.7 μM. | 25919052 | ||
| PSN1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human PSN1 cells after 72 hrs by WST8 assay, IC50 = 4.4 μM. | 25919052 | ||
| MDA-MB-231 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MDA-MB-231 cells after 72 hrs by WST8 assay, IC50 = 5.2 μM. | 25919052 | ||
| HeLa | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HeLa cells after 72 hrs by WST8 assay, IC50 = 5.8 μM. | 25919052 | ||
| TIG3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human TIG3 cells after 72 hrs by WST8 assay, IC50 = 8.4 μM. | 25919052 | ||
| HeLa | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HeLa cells assessed as inhibition of cell proliferation incubated for 48 hrs by CCK8 assay, IC50 = 8.93 μM. | 25959813 | ||
| MCF7 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MCF7 cells assessed as inhibition of cell proliferation incubated for 48 hrs by CCK8 assay, IC50 = 9.56 μM. | 25959813 | ||
| Bel7402 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human Bel7402 cells assessed as inhibition of cell proliferation incubated for 48 hrs by CCK8 assay, IC50 = 19.91 μM. | 25959813 | ||
| NIH/3T3 | Antiproliferative assay | 48 hrs | Antiproliferative activity against mouse NIH/3T3 cells assessed as inhibition of cell proliferation incubated for 48 hrs by CCK8 assay, IC50 = 22.36 μM. | 25959813 | ||
| A549 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human A549 cells assessed as inhibition of cell proliferation incubated for 48 hrs by CCK8 assay, IC50 = 40.38 μM. | 25959813 | ||
| HepG2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 cells after 48 hrs by MTT assay, IC50 = 8 μM. | 25969171 | ||
| L1210 | Cytotoxicity assay | Cytotoxicity against mouse L1210 cells assessed as inhibition of cell proliferation, IC50 = 0.33 μM. | 26001344 | |||
| HeLa | Cytotoxicity assay | Cytotoxicity against human HeLa cells assessed as inhibition of cell proliferation, IC50 = 0.54 μM. | 26001344 | |||
| CEM | Cytotoxicity assay | Cytotoxicity against human CEM cells assessed as inhibition of cell proliferation, IC50 = 18 μM. | 26001344 | |||
| PC3 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human PC3 cells assessed as growth inhibition after 48 hrs by sulphorhodamine B assay, IC50 = 2.2 μM. | 26222449 | ||
| HCT116 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HCT116 cells assessed as growth inhibition after 48 hrs by sulphorhodamine B assay, IC50 = 4.5 μM. | 26222449 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells assessed as growth inhibition after 48 hrs by sulphorhodamine B assay, IC50 = 4.9 μM. | 26222449 | ||
| HCT8 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT8 cells after 72 hrs by MTT assay, IC50 = 6.3 μM. | 26227777 | ||
| Bel7402 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human Bel7402 cells after 72 hrs by MTT assay, IC50 = 10.07 μM. | 26227777 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells after 72 hrs by MTT assay, IC50 = 14.15 μM. | 26227777 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells after 72 hrs by MTT assay, IC50 = 16.42 μM. | 26227777 | ||
| A549 | Anticancer assay | 48 hrs | Anticancer activity against human A549 cells assessed as reduction in cell growth incubated for 48 hrs by MTT assay, IC50 = 2.13 μM. | 26231080 | ||
| MCF7 | Anticancer assay | 48 hrs | Anticancer activity against human MCF7 cells assessed as reduction in cell growth incubated for 48 hrs by MTT assay, IC50 = 2.36 μM. | 26231080 | ||
| HeLa | Anticancer assay | 48 hrs | Anticancer activity against human HeLa cells assessed as reduction in cell growth incubated for 48 hrs by MTT assay, IC50 = 4.6 μM. | 26231080 | ||
| HaCaT | Cytotoxicity assay | 48 hrs | Cytotoxic activity against human HaCaT cells assessed as reduction in cell growth incubated for 48 hrs by MTT assay, IC50 = 15.26 μM. | 26231080 | ||
| Hep3B | Cytotoxicity assay | 2 days | Cytotoxicity against human Hep3B cells assessed as induction of cell death incubated for 2 days by MTT assay, IC50 = 1.5 μM. | 26259802 | ||
| Bcap37 | Cytotoxicity assay | 2 days | Cytotoxicity against human Bcap37 cells assessed as induction of cell death incubated for 2 days by MTT assay, IC50 = 5.58 μM. | 26259802 | ||
| MCF7 | Cytotoxicity assay | 2 days | Cytotoxicity against human MCF7 cells assessed as induction of cell death incubated for 2 days by MTT assay, IC50 = 11.5 μM. | 26259802 | ||
| HepG2 | Cytotoxicity assay | 2 days | Cytotoxicity against human HepG2 cells assessed as induction of cell death incubated for 2 days by MTT assay, IC50 = 40.34 μM. | 26259802 | ||
| A549 | Cytotoxicity assay | 2 days | Cytotoxicity against human A549 cells assessed as induction of cell death incubated for 2 days by MTT assay, IC50 = 44.05 μM. | 26259802 | ||
| SW620 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SW620 cells incubated for 72 hrs by MTT assay, IC50 = 0.79 μM. | 26291038 | ||
| MIAPaCa2 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MIAPaCa2 cells incubated for 72 hrs by MTT assay, IC50 = 11.67 μM. | 26291038 | ||
| MCF7 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MCF7 cells after 48 hrs by MTT assay, IC50 = 5.012 μM. | 26301558 | ||
| DU145 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human DU145 cells after 48 hrs by MTT assay, IC50 = 6.072 μM. | 26301558 | ||
| A549 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human A549 cells after 48 hrs by MTT assay, IC50 = 7.244 μM. | 26301558 | ||
| HeLa | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HeLa cells after 48 hrs by MTT assay, IC50 = 8.317 μM. | 26301558 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MCF7 cells after 48 hrs by MTT assay, IC50 = 19.39 μM. | 26363869 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells after 48 hrs by MTT assay, IC50 = 22.28 μM. | 26363869 | ||
| PC3 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human PC3 cells after 48 hrs by MTT assay, IC50 = 22.63 μM. | 26363869 | ||
| HepG2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 cells after 48 hrs by MTT assay, IC50 = 46.83 μM. | 26363869 | ||
| HT-29 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HT-29 cells assessed as growth inhibition after 48 hrs by WST-1 assay, IC50 = 15.83 μM. | 26413725 | ||
| PA1 | Anticancer assay | 48 hrs | Anticancer activity against human PA1 cells after 48 hrs by MTT assay, IC50 = 7.59 μM. | 26413726 | ||
| BT549 | Anticancer assay | 48 hrs | Anticancer activity against human BT549 cells after 48 hrs by MTT assay, IC50 = 19.82 μM. | 26413726 | ||
| DU145 | Anticancer assay | 48 hrs | Anticancer activity against human DU145 cells after 48 hrs by MTT assay, IC50 = 39.17 μM. | 26413726 | ||
| MDA-MB-231 | Anticancer assay | 48 hrs | Anticancer activity against human MDA-MB-231 cells after 48 hrs by MTT assay, IC50 = 45.43 μM. | 26413726 | ||
| SH-SY5Y | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SH-SY5Y cells after 72 hrs by MTT-based Mosmann assay relative to control, GI50 = 1.5 μM. | 26539626 | ||
| Caco2 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human Caco2 cells after 72 hrs by MTT-based Mosmann assay relative to control, GI50 = 2.2 μM. | 26539626 | ||
| A549 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A549 cells after 72 hrs by MTT assay, IC50 = 6.76 μM. | 26595184 | ||
| SGC7901 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SGC7901 cells after 72 hrs by MTT assay, IC50 = 11.9 μM. | 26595184 | ||
| MDA-MB-435S | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MDA-MB-435S cells after 72 hrs by MTT assay, IC50 = 15.19 μM. | 26595184 | ||
| L02 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human L02 cells after 72 hrs by MTT assay, IC50 = 25.9 μM. | 26595184 | ||
| PC3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human PC3 cells after 72 hrs by MTT assay, IC50 = 42.5 μM. | 26595184 | ||
| PANC1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human PANC1 cells after 72 hrs by WST-8 assay, IC50 = 0.4 μM. | 26606140 | ||
| MDA-MB-231 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MDA-MB-231 cells after 72 hrs by WST-8 assay, IC50 = 1.1 μM. | 26606140 | ||
| HeLa | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HeLa cells after 72 hrs by WST-8 assay, IC50 = 6.4 μM. | 26606140 | ||
| PSN1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human PSN1 cells after 72 hrs by WST-8 assay, IC50 = 8.7 μM. | 26606140 | ||
| A549 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A549 cells after 72 hrs by WST-8 assay, IC50 = 9 μM. | 26606140 | ||
| MCF7 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MCF7 cells after 72 hrs by MTT assay, IC50 = 0.09 μM. | 26617970 | ||
| SW620 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SW620 cells after 72 hrs by MTT assay, IC50 = 0.79 μM. | 26617970 | ||
| A549 | Cytotoxicity assay | Cytotoxicity against human A549 cells assessed as cell growth inhibition by MTT assay, IC50 = 1.1 μM. | 26629861 | |||
| Neuro2a | Cytotoxicity assay | Cytotoxicity against mouse Neuro2a cells assessed as cell growth inhibition by MTT assay, IC50 = 1.2 μM. | 26629861 | |||
| HeLa | Cytotoxicity assay | Cytotoxicity against human HeLa cells assessed as cell growth inhibition by MTT assay, IC50 = 1.5 μM. | 26629861 | |||
| COLO205 | Cytotoxicity assay | Cytotoxicity against human COLO205 cells assessed as cell growth inhibition by MTT assay, IC50 = 1.8 μM. | 26629861 | |||
| A549 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A549 cells after 72 hrs by MTS assay, IC50 = 3.52 μM. | 26703795 | ||
| HCT116 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT116 cells after 72 hrs by MTS assay, IC50 = 5.53 μM. | 26703795 | ||
| HT-29 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HT-29 cells after 72 hrs by MTS assay, IC50 = 24.5 μM. | 26703795 | ||
| MCF7 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MCF7 cells after 72 hrs by MTS assay, IC50 = 32.18 μM. | 26703795 | ||
| HeLa | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HeLa cells after 72 hrs by MTS assay, IC50 = 43.71 μM. | 26703795 | ||
| SKOV3 | Cytotoxicity assay | 48 hrs | Cytotoxic activity against human SKOV3 cells assessed as inhibition of cell growth after 48 hrs by MTT assay, IC50 = 22.43 μM. | 26706349 | ||
| HCT116 | Cytotoxicity assay | 48 hrs | Cytotoxic activity against human HCT116 cells assessed as inhibition of cell growth after 48 hrs by MTT assay, IC50 = 24.58 μM. | 26706349 | ||
| Bel7404 | Cytotoxicity assay | 48 hrs | Cytotoxic activity against human Bel7404 cells assessed as inhibition of cell growth after 48 hrs by MTT assay, IC50 = 30.23 μM. | 26706349 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxic activity against human A549 cells assessed as inhibition of cell growth after 48 hrs by MTT assay, IC50 = 31.33 μM. | 26706349 | ||
| NCI-H460 | Cytotoxicity assay | 48 hrs | Cytotoxic activity against human NCI-H460 cells assessed as inhibition of cell growth after 48 hrs by MTT assay, IC50 = 35.52 μM. | 26706349 | ||
| MDST8 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MDST8 cells after 72 hrs by CCK8 assay, IC50 = 0.064 μM. | 26725029 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by CCK8 assay, IC50 = 0.52 μM. | 26725029 | ||
| KM12 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human KM12 cells after 48 hrs by sulforhodamine B assay, IC50 = 14.8 μM. | 26727215 | ||
| HT-29 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HT-29 cells after 48 hrs by sulforhodamine B assay, IC50 = 14.8 μM. | 26727215 | ||
| HCC2998 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HCC2998 cells after 48 hrs by sulforhodamine B assay, IC50 = 14.8 μM. | 26727215 | ||
| COLO205 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human COLO205 cells after 48 hrs by sulforhodamine B assay, IC50 = 14.8 μM. | 26727215 | ||
| HCT116 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HCT116 cells after 48 hrs by sulforhodamine B assay, IC50 = 14.8 μM. | 26727215 | ||
| HCT15 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HCT15 cells after 48 hrs by sulforhodamine B assay, IC50 = 14.8 μM. | 26727215 | ||
| SW620 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human SW620 cells after 48 hrs by sulforhodamine B assay, IC50 = 14.8 μM. | 26727215 | ||
| MCF7 | Antiproliferative assay | 96 hrs | Antiproliferative activity against human MCF7 cells assessed as cellular DNA content after 96 hrs by CyQUANT NF fluorescence assay, IC50 = 0.9 μM. | 26731300 | ||
| PC3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human PC3 cells assessed as cellular DNA content after 72 hrs by CyQUANT NF fluorescence assay, IC50 = 4.9 μM. | 26731300 | ||
| HT-29 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HT-29 cells assessed as cellular DNA content after 72 hrs by CyQUANT NF fluorescence assay, IC50 = 5.3 μM. | 26731300 | ||
| T84 | Antiproliferative assay | 96 hrs | Antiproliferative activity against human T84 cells assessed as cellular DNA content after 96 hrs by CyQUANT NF fluorescence assay, IC50 = 6.2 μM. | 26731300 | ||
| PC3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human PC3 cells assessed as growth inhibition after 72 hrs by MTT assay, IC50 = 1.44 μM. | 26735909 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells assessed as growth inhibition after 72 hrs by MTT assay, IC50 = 3.47 μM. | 26735909 | ||
| U87MG | Cytotoxicity assay | 72 hrs | Cytotoxicity against human U87MG cells assessed as growth inhibition after 72 hrs by MTT assay, IC50 = 3.61 μM. | 26735909 | ||
| L929 | Antiproliferative assay | 48 hrs | Antiproliferative activity against mouse L929 cells after 48 hrs by CCK8 assay, IC50 = 2.98 μM. | 26786698 | ||
| SMMC7721 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human SMMC7721 cells after 48 hrs by CCK8 assay, IC50 = 5.62 μM. | 26786698 | ||
| A549 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human A549 cells after 48 hrs by CCK8 assay, IC50 = 8.13 μM. | 26786698 | ||
| HeLa | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HeLa cells after 48 hrs by CCK8 assay, IC50 = 17.21 μM. | 26786698 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells after 48 hrs by MTT assay, IC50 = 1.4 μM. | 26854375 | ||
| THP1 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human THP1 cells after 48 hrs by MTT assay, IC50 = 1.7 μM. | 26854375 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MCF7 cells after 48 hrs by MTT assay, IC50 = 2 μM. | 26854375 | ||
| HCT116 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HCT116 cells after 48 hrs by MTT assay, IC50 = 2.3 μM. | 26854375 | ||
| HCT8 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HCT8 cells assessed as growth inhibition after 48 hrs by MTT assay, IC50 = 32.31 μM. | 26873416 | ||
| WI38 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human WI38 cells assessed as growth inhibition after 48 hrs by MTT assay, IC50 = 38.91 μM. | 26873416 | ||
| HepG2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 cells assessed as growth inhibition after 48 hrs by MTT assay, IC50 = 41.62 μM. | 26873416 | ||
| Hep3B | Cytotoxicity assay | 48 hrs | Cytotoxicity against human Hep3B cells after 48 hrs by MTT assay, IC50 = 10.53 μM. | 26883148 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells after 48 hrs by MTT assay, IC50 = 34.27 μM. | 26883148 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MCF7 cells after 48 hrs by MTT assay, IC50 = 42.78 μM. | 26883148 | ||
| Bcap37 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human Bcap37 cells after 48 hrs by MTT assay, IC50 = 47.09 μM. | 26883148 | ||
| HepG2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 cells after 48 hrs by MTT assay, IC50 = 49.72 μM. | 26883148 | ||
| MCF7 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MCF7 cells assessed as cell viability after 72 hrs by MTT assay, IC50 = 26.14 μM. | 26922223 | ||
| HeLa | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HeLa cells assessed as cell viability after 72 hrs by MTT assay, IC50 = 26.18 μM. | 26922223 | ||
| HL60 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HL60 cells assessed as cell viability after 72 hrs by MTT assay, IC50 = 27.43 μM. | 26922223 | ||
| HCT116 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT116 cells assessed as cell viability after 72 hrs by MTT assay, IC50 = 38.3 μM. | 26922223 | ||
| HepG2 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HepG2 cells assessed as cell viability after 72 hrs by MTT assay, IC50 = 49.02 μM. | 26922223 | ||
| HT-29 | Anticancer assay | 72 hrs | Anticancer activity against human HT-29 cells assessed as inhibition of cell growth after 72 hrs by MTT assay, IC50 = 6.3 μM. | 26922233 | ||
| RKO | Anticancer assay | 72 hrs | Anticancer activity against human RKO cells assessed as inhibition of cell growth after 72 hrs by MTT assay, IC50 = 6.8 μM. | 26922233 | ||
| HT-29 | Anticancer assay | 48 hrs | Anticancer activity against human HT-29 cells assessed as inhibition of cell growth after 48 hrs by MTT assay, IC50 = 25 μM. | 26922233 | ||
| HCT116 | Anticancer assay | 72 hrs | Anticancer activity against human HCT116 cells assessed as inhibition of cell growth after 72 hrs by MTT assay, IC50 = 27.8 μM. | 26922233 | ||
| RKO | Anticancer assay | 48 hrs | Anticancer activity against human RKO cells assessed as inhibition of cell growth after 48 hrs by MTT assay, IC50 = 41.1 μM. | 26922233 | ||
| KB | Cytotoxicity assay | 72 hrs | Cytotoxicity against human KB cells after 72 hrs by SRB assay, IC50 = 4.84 μM. | 27073055 | ||
| HeLa | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HeLa cells after 72 hrs by SRB assay, IC50 = 6.23 μM. | 27073055 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7cells after 72 hrs by SRB assay, IC50 = 6.53 μM. | 27073055 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells after 72 hrs by SRB assay, IC50 = 6.6 μM. | 27073055 | ||
| HDF | Cytotoxicity assay | 72 hrs | Cytotoxicity against HDF cells after 72 hrs by SRB assay, IC50 = 7.02 μM. | 27073055 | ||
| 143B | Cytotoxicity assay | 72 hrs | Cytotoxicity against human 143B cells after 72 hrs by SRB assay, IC50 = 13.01 μM. | 27073055 | ||
| thymidine kinase deficient 143B | Cytotoxicity assay | 72 hrs | Cytotoxicity against human thymidine kinase deficient 143B cells after 72 hrs by SRB assay, IC50 = 16.31 μM. | 27073055 | ||
| U87MG | Cytotoxicity assay | 72 hrs | Cytotoxicity against human U87MG cells assessed as reduction in cell viability after 72 hrs by Z2 coulter counter method, IC50 = 18.25 μM. | 27081742 | ||
| PC3 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human PC3 cells after 48 hrs by MTT assay, IC50 = 2.76 μM. | 27092413 | ||
| HT-29 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HT-29 cells after 48 hrs by MTT assay, IC50 = 2.8 μM. | 27092413 | ||
| U87MG | Antiproliferative assay | 48 hrs | Antiproliferative activity against human U87MG cells after 48 hrs by MTT assay, IC50 = 3.65 μM. | 27092413 | ||
| A549 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human A549 cells after 48 hrs by MTT assay, IC50 = 3.98 μM. | 27092413 | ||
| HaCaT | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HaCaT cells after 48 hrs by MTT assay, IC50 = 16.15 μM. | 27092413 | ||
| MGC803 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MGC803 cells assessed as cell growth inhibition after 72 hrs by MTT assay, IC50 = 1.25 μM. | 27105028 | ||
| EC109 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human EC109 cells assessed as cell growth inhibition after 72 hrs by MTT assay, IC50 = 3.17 μM. | 27105028 | ||
| SH-SY5Y | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SH-SY5Y cells assessed as cell growth inhibition after 72 hrs by MTT assay, IC50 = 3.26 μM. | 27105028 | ||
| U87 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human U87 cells assessed as cell growth inhibition after 72 hrs by MTT assay, IC50 = 5.61 μM. | 27105028 | ||
| MCF7 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MCF7 cells assessed as cell growth inhibition after 72 hrs by MTT assay, IC50 = 7.61 μM. | 27105028 | ||
| MDA-MB-231 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MDA-MB-231 cells after 48 hrs by MTT assay, IC50 = 24.6 μM. | 27128180 | ||
| MCF7 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MCF7 cells after 48 hrs by MTT assay, IC50 = 30.41 μM. | 27128180 | ||
| A549 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human A549 cells after 48 hrs by MTT assay, IC50 = 35.41 μM. | 27128180 | ||
| HepG2 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HepG2 cells after 48 hrs by MTT assay, IC50 = 46.83 μM. | 27128180 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MCF7 cells after 48 hrs by MTT assay, IC50 = 13.5 μM. | 27133593 | ||
| Bcap37 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human Bcap37 cells after 48 hrs by MTT assay, IC50 = 15.47 μM. | 27133593 | ||
| Hep3B | Cytotoxicity assay | 48 hrs | Cytotoxicity against human Hep3B cells after 48 hrs by MTT assay, IC50 = 17.53 μM. | 27133593 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells after 48 hrs by MTT assay, IC50 = 21.58 μM. | 27133593 | ||
| U251 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human U251 cells after 48 hrs by MTT assay, IC50 = 24.69 μM. | 27133593 | ||
| HepG2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 cells after 48 hrs by MTT assay, IC50 = 25.17 μM. | 27133593 | ||
| LNCAP | Cytotoxicity assay | 24 hrs | Cytotoxicity against human LNCAP cells assessed as growth inhibition after 24 hrs by sulforhodamine B assay, IC50 = 0.03 μM. | 27156770 | ||
| PC3 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human PC3 cells assessed as growth inhibition after 24 hrs by sulforhodamine B assay, IC50 = 0.03 μM. | 27156770 | ||
| Bcap37 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human Bcap37 cells assessed as growth inhibition after 48 hrs by MTT assay, IC50 = 5.18 μM. | 27189676 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MCF7 cells assessed as growth inhibition after 48 hrs by MTT assay, IC50 = 11.5 μM. | 27189676 | ||
| Hep3B | Cytotoxicity assay | 48 hrs | Cytotoxicity against human Hep3B cells assessed as growth inhibition after 48 hrs by MTT assay, IC50 = 22.99 μM. | 27189676 | ||
| HepG2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 cells assessed as growth inhibition after 48 hrs by MTT assay, IC50 = 40.34 μM. | 27189676 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells assessed as growth inhibition after 48 hrs by MTT assay, IC50 = 44.05 μM. | 27189676 | ||
| PA1 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human PA1 cells assessed as decrease in cell viability after 48 hrs by MTT assay, IC50 = 8.51 μM. | 27210438 | ||
| U87MG | Cytotoxicity assay | 48 hrs | Cytotoxicity against human U87MG cells assessed as decrease in cell viability after 48 hrs by MTT assay, IC50 = 15.48 μM. | 27210438 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MCF7 cells assessed as decrease in cell viability after 48 hrs by MTT assay, IC50 = 32.27 μM. | 27210438 | ||
| LNCAP | Cytotoxicity assay | 48 hrs | Cytotoxicity against human LNCAP cells assessed as decrease in cell viability after 48 hrs by MTT assay, IC50 = 47.18 μM. | 27210438 | ||
| MCF7 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human MCF7 cells assessed as cell survivability after 24 hrs by crystal violet staining based microscopic analysis, IC50 = 11.1 μM. | 27234893 | ||
| HepG2 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HepG2 cells measured after 48 hrs by MTT assay, IC50 = 46.83 μM. | 27287368 | ||
| A375-S2 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human A375-S2 cells assessed as inhibition of cell viability after 48 hrs by MTT reduction assay, IC50 = 1.91 μM. | 27295506 | ||
| ACHN | Antiproliferative assay | 48 hrs | Antiproliferative activity against human ACHN cells assessed as inhibition of cell viability after 48 hrs by MTT reduction assay, IC50 = 2.73 μM. | 27295506 | ||
| 22Rv1 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human 22Rv1 cells assessed as inhibition of cell viability after 48 hrs by MTT reduction assay, IC50 = 3.83 μM. | 27295506 | ||
| C4-2B | Antiproliferative assay | 48 hrs | Antiproliferative activity against human C4-2B cells assessed as inhibition of cell viability after 48 hrs by MTT reduction assay, IC50 = 5.64 μM. | 27295506 | ||
| A498 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human A498 cells assessed as inhibition of cell viability after 48 hrs by MTT reduction assay, IC50 = 8.83 μM. | 27295506 | ||
| A549 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A549 cells assessed as reduction in cell viability after 72 hrs by WST-8 assay, IC50 = 2.8 μM. | 27311895 | ||
| MDA-MB-231 | Antiproliferative assay | 72 hrs | Antiproliferation activity against human MDA-MB-231 cells assessed as reduction in cell viability after 72 hrs by WST-8 assay, IC50 = 5.2 μM. | 27311895 | ||
| HeLa | Antiproliferative assay | 72 hrs | Antiproliferation activity against human HeLa cells assessed as reduction in cell viability after 72 hrs by WST-8 assay, IC50 = 5.8 μM. | 27311895 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MCF7 cells assessed as reduction in cell viability after 48 hrs by MTT assay, IC50 = 3 μM. | 27343853 | ||
| WI38 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human WI38 cells assessed as reduction in cell viability after 48 hrs by MTT assay, IC50 = 4 μM. | 27343853 | ||
| HepG2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 cells assessed as reduction in cell viability after 48 hrs by MTT assay, IC50 = 8 μM. | 27343853 | ||
| UO31 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human UO31 cells measured after 48 hrs by SRB assay, GI50 = 6.76083 μM. | 27391135 | ||
| U251 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human U251 cells measured after 48 hrs by SRB assay, GI50 = 42.6579 μM. | 27391135 | ||
| HCT116 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HCT116 cells assessed as cell growth inhibition after 48 hrs by MTT assay, IC50 = 15.38 μM. | 27448774 | ||
| MDA-MB-231 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MDA-MB-231 cells assessed as cell growth inhibition after 48 hrs by MTT assay, IC50 = 35.91 μM. | 27448774 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells assessed as cell growth inhibition after 48 hrs by MTT assay, IC50 = 39.86 μM. | 27448774 | ||
| Caco2 | Growth inhibition assay | 72 hrs | Growth inhibition of human Caco2 cells measured after 72 hrs by MTT assay, GI50 = 3.3 μM. | 27504537 | ||
| MCF7 | Growth inhibition assay | 72 hrs | Growth inhibition of human MCF7 cells measured after 72 hrs by MTT assay, GI50 = 3.6 μM. | 27504537 | ||
| PC3 | Growth inhibition assay | 72 hrs | Growth inhibition of human PC3 cells measured after 72 hrs by MTT assay, GI50 = 4.1 μM. | 27504537 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells measured after 72 hrs by MTT assay, IC50 = 6.8 μM. | 27537924 | ||
| SGC7901 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human SGC7901 cells measured after 72 hrs by MTT assay, IC50 = 11.9 μM. | 27537924 | ||
| MDA-MB-435S | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MDA-MB-435S cells measured after 72 hrs by MTT assay, IC50 = 15.2 μM. | 27537924 | ||
| PC3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human PC3 cells measured after 72 hrs by MTT assay, IC50 = 42.5 μM. | 27537924 | ||
| colon cancer cell | Growth inhibition assay | 48 hrs | Growth inhibition of human colon cancer cells after 48 hrs by SRB assay, GI50 = 8.4 μM. | 27554444 | ||
| leukemia cell | Growth inhibition assay | 48 hrs | Growth inhibition of human leukemia cells after 48 hrs by SRB assay, GI50 = 15.1 μM. | 27554444 | ||
| prostate cancer cell | Growth inhibition assay | 48 hrs | Growth inhibition of human prostate cancer cells after 48 hrs by SRB assay, GI50 = 22.7 μM. | 27554444 | ||
| renal cancer cell | Growth inhibition assay | 48 hrs | Growth inhibition of human renal cancer cells after 48 hrs by SRB assay, GI50 = 45.6 μM. | 27554444 | ||
| DU145 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human DU145 cells assessed as reduction in cell viability after 48 hrs by MTT assay, IC50 = 26.1 μM. | 27561718 | ||
| HeLa | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HeLa cells assessed as reduction in cell viability after 48 hrs by MTT assay, IC50 = 28.2 μM. | 27561718 | ||
| IOSE-144 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human IOSE-144 cells assessed as reduction in cell viability after 48 hrs by MTT assay, IC50 = 36.1 μM. | 27561718 | ||
| HepG2 | Cytotoxicity assay | Cytotoxicity against human HepG2 cells assessed as cell growth inhibition by MTT assay, IC50 = 1.3 μM. | 27561719 | |||
| DU145 | Cytotoxicity assay | Cytotoxicity against human DU145 cells assessed as cell growth inhibition by MTT assay, IC50 = 1.6 μM. | 27561719 | |||
| MDA-MB-231 | Cytotoxicity assay | Cytotoxicity against human MDA-MB-231 cells assessed as cell growth inhibition by MTT assay, IC50 = 1.8 μM. | 27561719 | |||
| HeLa | Cytotoxicity assay | Cytotoxicity against human HeLa cells assessed as cell growth inhibition by MTT assay, IC50 = 1.9 μM. | 27561719 | |||
| SW780 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human SW780 cells assessed as growth inhibition after 72 hrs by CCK8 assay, IC50 = 6.99 μM. | 27597411 | ||
| MGC803 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MGC803 cells assessed as growth inhibition after 72 hrs by CCK8 assay, IC50 = 10.64 μM. | 27597411 | ||
| HL-7702 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HL-7702 cells assessed as growth inhibition after 72 hrs by CCK8 assay, IC50 = 12.85 μM. | 27597411 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells assessed as growth inhibition after 72 hrs by CCK8 assay, IC50 = 13.19 μM. | 27597411 | ||
| GES-1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human GES-1 cells assessed as growth inhibition after 72 hrs by CCK8 assay, IC50 = 13.75 μM. | 27597411 | ||
| TE1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human TE1 cells assessed as growth inhibition after 72 hrs by CCK8 assay, IC50 = 17.32 μM. | 27597411 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells assessed as cell growth inhibition after 48 hrs by MTT assay, IC50 = 30.47 μM. | 27614408 | ||
| MDA-MB-231 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MDA-MB-231 cells assessed as cell growth inhibition after 48 hrs by MTT assay, IC50 = 35.98 μM. | 27614408 | ||
| DU145 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human DU145 cells assessed as cell growth inhibition after 48 hrs by MTT assay, IC50 = 40.58 μM. | 27614408 | ||
| PC3 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human PC3 cells assessed as cell growth inhibition after 48 hrs by MTT assay, IC50 = 45.32 μM. | 27614408 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MCF7 cells assessed as growth inhibition measured after 48 hrs by MTT assay, GI50 = 4.5 μM. | 27688184 | ||
| HepG2 | Function assay | 48 hrs | Hepatotoxicity against human HepG2 cells assessed as growth inhibition measured after 48 hrs by MTT assay, GI50 = 7 μM. | 27688184 | ||
| U87 | Function assay | 48 hrs | Neurotoxicity against human U87 cells assessed as growth inhibition measured after 48 hrs by MTT assay, GI50 = 13 μM. | 27688184 | ||
| HCT116 | Growth inhibition assay | 72 hrs | Growth inhibition of human HCT116 cells after 72 hrs by MTS assay, IC50 = 2.5 μM. | 27704811 | ||
| HeLa | Cytotoxicity assay | Cytotoxicity against human HeLa cells assessed as cell growth inhibition by MTT assay, IC50 = 1.6 μM. | 27720296 | |||
| PC3 | Cytotoxicity assay | Cytotoxicity against human PC3 cells assessed as cell growth inhibition by MTT assay, IC50 = 1.8 μM. | 27720296 | |||
| HepG2 | Cytotoxicity assay | Cytotoxicity against human HepG2 cells assessed as cell growth inhibition by MTT assay, IC50 = 1.8 μM. | 27720296 | |||
| MDA-MB-231 | Cytotoxicity assay | Cytotoxicity against human MDA-MB-231 cells assessed as cell growth inhibition by MTT assay, IC50 = 1.9 μM. | 27720296 | |||
| L1210 | Antiproliferative assay | 48 hrs | Anti-proliferative activity against mouse L1210 cells measured after 48 hrs by coulter counter method, IC50 = 0.33 μM. | 27750154 | ||
| HeLa | Antiproliferative assay | 4 days | Anti-proliferative activity against human HeLa cells measured after 4 days by coulter counter method, IC50 = 0.54 μM. | 27750154 | ||
| CEM | Antiproliferative assay | 72 hrs | Anti-proliferative activity against human CEM cells measured after 72 hrs by coulter counter method, IC50 = 18 μM. | 27750154 | ||
| PC9 | Cytotoxicity assay | Cytotoxicity against human PC9 cells assessed as growth inhibition by MTT assay, IC50 = 1.99 μM. | 27769034 | |||
| MGC803 | Cytotoxicity assay | Cytotoxicity against human MGC803 cells assessed as growth inhibition by MTT assay, IC50 = 10.14 μM. | 27769034 | |||
| A549 | Cytotoxicity assay | Cytotoxicity against human A549 cells assessed as growth inhibition by MTT assay, IC50 = 13.95 μM. | 27769034 | |||
| EC109 | Cytotoxicity assay | Cytotoxicity against human EC109 cells assessed as growth inhibition by MTT assay, IC50 = 25.99 μM. | 27769034 | |||
| MGC803 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MGC803 cells after 72 hrs by MTT assay, IC50 = 7.35 μM. | 27771599 | ||
| MCF7 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MCF7 cells after 72 hrs by MTT assay, IC50 = 9.25 μM. | 27771599 | ||
| EC109 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human EC109 cells after 72 hrs by MTT assay, IC50 = 10.59 μM. | 27771599 | ||
| HUCCA | Cytotoxicity assay | 24 hrs | Cytotoxicity against HUCCA cells assessed as decrease in cell viability after 24 hrs by MTT assay, IC50 = 13.4 μM. | 27825763 | ||
| QBC939 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human QBC939 cells assessed as decrease in cell viability after 24 hrs by MTT assay, IC50 = 16.1 μM. | 27825763 | ||
| SW620 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SW620 cells after 72 hrs by MTT assay, IC50 = 0.08 μM. | 27875779 | ||
| CFPAC-1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human CFPAC-1 cells after 72 hrs by MTT assay, IC50 = 0.14 μM. | 27875779 | ||
| WI38 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human WI38 cells after 72 hrs by MTT assay, IC50 = 0.94 μM. | 27875779 | ||
| A549 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A549 cells after 72 hrs by MTT assay, IC50 = 2.8 μM. | 27875779 | ||
| HeLa | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HeLa cells after 72 hrs by MTT assay, IC50 = 8.81 μM. | 27875779 | ||
| HepG2 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HepG2 cells after 72 hrs by MTT assay, IC50 = 8.9 μM. | 27875779 | ||
| Hep3B | Cytotoxicity assay | Cytotoxicity against human Hep3B cells assessed as cell growth inhibition by MTT assay, IC50 = 9.36 μM. | 27913179 | |||
| A549 | Cytotoxicity assay | Cytotoxicity against human A549 cells assessed as cell growth inhibition by MTT assay, IC50 = 16.63 μM. | 27913179 | |||
| WISH | Cytotoxicity assay | 24 hrs | Cytotoxicity against human WISH cells assessed as reduction in cell viability after 24 hrs by MTT assay, IC50 = 7.1 μM. | 27951487 | ||
| WI38 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human WI38 cells assessed as reduction in cell viability after 24 hrs by MTT assay, IC50 = 9.3 μM. | 27951487 | ||
| A549 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A549 cells after 72 hrs by MTT assay, IC50 = 1.16 μM. | 27987485 | ||
| MDA-MB-231 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MDA-MB-231 cells after 72 hrs by MTT assay, IC50 = 5 μM. | 27987485 | ||
| HepG2 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HepG2 cells after 72 hrs by MTT assay, IC50 = 5 μM. | 27987485 | ||
| MCF7 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MCF7 cells after 72 hrs by MTT assay, IC50 = 7.12 μM. | 27987485 | ||
| Hep3B | Cytotoxicity assay | 48 hrs | Cytotoxic activity against human Hep3B cells assessed as growth inhibition after 48 hrs by MTT assay, IC50 = 10.53 μM. | 27993516 | ||
| HepG2 | Cytotoxicity assay | 48 hrs | Cytotoxic activity against human HepG2 cells assessed as growth inhibition after 48 hrs by MTT assay, IC50 = 32.6 μM. | 27993516 | ||
| HepG2 | Growth inhibition assay | 24 hrs | Growth inhibition of human HepG2 cells after 24 hrs by MTT assay, IC50 = 32.87 μM. | 28011220 | ||
| PA1 | Growth inhibition assay | 24 hrs | Growth inhibition of human PA1 cells after 24 hrs by MTT assay, IC50 = 39.5 μM. | 28011220 | ||
| HCT15 | Growth inhibition assay | 24 hrs | Growth inhibition of human HCT15 cells after 24 hrs by MTT assay, IC50 = 45.01 μM. | 28011220 | ||
| PC3 | Cytotoxicity assay | 72 hrs | Cytotoxic activity against human PC3 cells assessed as reduction in cell viability after 72 hrs by SRB assay, IC50 = 15.31 μM. | 28011223 | ||
| SKOV3 | Cytotoxicity assay | 72 hrs | Cytotoxic activity against human SKOV3 cells assessed as reduction in cell viability after 72 hrs by SRB assay, IC50 = 23.11 μM. | 28011223 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxic activity against human A549 cells assessed as reduction in cell viability after 72 hrs by SRB assay, IC50 = 32.15 μM. | 28011223 | ||
| HepG2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 cells assessed as decrease in cell proliferation after 48 hrs by MTT colorimetric assay, IC50 = 10.17 μM. | 28038327 | ||
| Hep3B | Cytotoxicity assay | 48 hrs | Cytotoxicity against human Hep3B cells assessed as decrease in cell proliferation after 48 hrs by MTT colorimetric assay, IC50 = 10.53 μM. | 28038327 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells assessed as decrease in cell proliferation after 48 hrs by MTT colorimetric assay, IC50 = 34.27 μM. | 28038327 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MCF7 cells assessed as decrease in cell proliferation after 48 hrs by MTT colorimetric assay, IC50 = 42.78 μM. | 28038327 | ||
| Bcap37 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human Bcap37 cells assessed as decrease in cell proliferation after 48 hrs by MTT colorimetric assay, IC50 = 47.1 μM. | 28038327 | ||
| U251 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human U251 cells assessed as decrease in cell proliferation after 48 hrs by MTT colorimetric assay, IC50 = 48.68 μM. | 28038327 | ||
| MGC803 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MGC803 cells assessed as inhibition of cell viability after 48 hrs by MTT assay, IC50 = 7.21 μM. | 28038329 | ||
| EC109 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human EC109 cells assessed as inhibition of cell viability after 48 hrs by MTT assay, IC50 = 10.3 μM. | 28038329 | ||
| PC3 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human PC3 cells assessed as inhibition of cell viability after 48 hrs by MTT assay, IC50 = 29.31 μM. | 28038329 | ||
| A549 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human A549 cells after 48 hrs by MTT assay, IC50 = 10.5 μM. | 28073676 | ||
| MCF7 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MCF7 cells after 48 hrs by MTT assay, IC50 = 19.2 μM. | 28073676 | ||
| HCT116 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HCT116 cells after 48 hrs by MTT assay, IC50 = 29.2 μM. | 28073676 | ||
| BGC823 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human BGC823 cells after 48 hrs by MTT assay, IC50 = 34.8 μM. | 28073676 | ||
| IOSE-144 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human IOSE-144 cells after 48 hrs by MTT assay, IC50 = 38.1 μM. | 28073676 | ||
| MCF7 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MCF7 cells after 48 hrs by MTT assay, IC50 = 26.14 μM. | 28110871 | ||
| HCT116 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HCT116 cells after 48 hrs by MTT assay, IC50 = 38.3 μM. | 28110871 | ||
| PANC1 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human PANC1 cells after 48 hrs by MTT assay, IC50 = 47.83 μM. | 28110871 | ||
| HepG2 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HepG2 cells after 48 hrs by MTT assay, IC50 = 49.02 μM. | 28110871 | ||
| BCG823 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human BCG823 cells assessed as reduction in cell viability after 48 hrs by MTT assay, IC50 = 10.1 μM. | 28117202 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells assessed as reduction in cell viability after 48 hrs by MTT assay, IC50 = 16.1 μM. | 28117202 | ||
| HepG2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 cells assessed as reduction in cell viability after 48 hrs by MTT assay, IC50 = 17.2 μM. | 28117202 | ||
| EC9706 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human EC9706 cells after 48 hrs by MTT assay, IC50 = 23.26 μM. | 28119200 | ||
| ECA109 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human ECA109 cells after 48 hrs by MTT assay, IC50 = 30.25 μM. | 28119200 | ||
| K562 | Growth inhibition assay | 48 hrs | Growth inhibition of human K562 cells after 48 hrs by WST1 assay, IC50 = 38.58 μM. | 28126436 | ||
| KKU-M214 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human KKU-M214 cells assessed as reduction in cell viability after 72 hrs by SRB assay, IC50 = 3.76 μM. | 28140592 | ||
| HL60 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HL60 cells after 48 hrs by MTS assay, IC50 = 1.62 μM. | 28152430 | ||
| NCI-H1299 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human NCI-H1299 cells after 48 hrs by MTS assay, IC50 = 8.91 μM. | 28152430 | ||
| MCF7 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MCF7 cells after 72 hrs by MTT assay, IC50 = 1.75 μM. | 28165738 | ||
| Bel7402 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human Bel7402 cells after 72 hrs by MTT assay, IC50 = 2.25 μM. | 28165738 | ||
| MGC803 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MGC803 cells after 72 hrs by MTT assay, IC50 = 3.12 μM. | 28165738 | ||
| K562 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human K562 cells after 72 hrs by MTT assay, IC50 = 3.54 μM. | 28165738 | ||
| HepG2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 cells assessed as growth inhibition after 48 hrs by MTT assay, IC50 = 10.53 μM. | 28174107 | ||
| Hep3B | Cytotoxicity assay | 48 hrs | Cytotoxicity against human Hep3B cells assessed as growth inhibition after 48 hrs by MTT assay, IC50 = 49.72 μM. | 28174107 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells assessed as growth inhibition after 72 hrs by SRB assay, IC50 = 1.4 μM. | 28219046 | ||
| HCT116 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT116 cells assessed as growth inhibition after 72 hrs by SRB assay, IC50 = 18.4 μM. | 28219046 | ||
| HuH7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HuH7 cells assessed as growth inhibition after 72 hrs by SRB assay, IC50 = 21 μM. | 28219046 | ||
| A549 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human A549 cells after 24 hrs by MTT assay, IC50 = 1.2 μM. | 28254697 | ||
| HeLa | Cytotoxicity assay | 24 hrs | Cytotoxicity against human HeLa cells after 24 hrs by MTT assay, IC50 = 1.4 μM. | 28254697 | ||
| HepG2 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human HepG2 cells after 24 hrs by MTT assay, IC50 = 1.4 μM. | 28254697 | ||
| COLO205 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human COLO205 cells after 24 hrs by MTT assay, IC50 = 1.9 μM. | 28254697 | ||
| HepG2 | Cytotoxicity assay | Cytotoxicity against human HepG2 cells by MTT assay, IC50 = 2.1 μM. | 28262527 | |||
| Hep3B | Cytotoxicity assay | Cytotoxicity against human Hep3B cells by MTT assay, IC50 = 2.66 μM. | 28262527 | |||
| Bel7402 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human Bel7402 cells after 72 hrs by CCK-8 assay, IC50 = 5.94 μM. | 28301815 | ||
| LO2 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human LO2 cells after 72 hrs by CCK-8 assay, IC50 = 32.546 μM. | 28301815 | ||
| HCT116 | Cytotoxicity assay | Cytotoxicity in human HCT116 cells assessed as reduction in cell viability by MTT assay, IC50 = 41 μM. | 28334654 | |||
| MCF7 | Cytotoxicity assay | Cytotoxicity in human MCF7 cells assessed as reduction in cell viability by MTT assay, IC50 = 41.5 μM. | 28334654 | |||
| HeLa | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HeLa cells after 48 hrs by MTT assay, IC50 = 9.02 μM. | 28363444 | ||
| HepG2 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HepG2 cells after 48 hrs by MTT assay, IC50 = 13.31 μM. | 28363444 | ||
| BGC823 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human BGC823 cells after 48 hrs by MTT assay, IC50 = 17.37 μM. | 28363444 | ||
| SW1116 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human SW1116 cells after 48 hrs by MTT assay, IC50 = 18.52 μM. | 28363444 | ||
| Hep3B | Cytotoxicity assay | Cytotoxicity against human Hep3B cells by MTT assay, IC50 = 11.9 μM. | 28371676 | |||
| MCF7 | Cytotoxicity assay | Cytotoxicity against human MCF7 cells by MTT assay, IC50 = 30 μM. | 28371676 | |||
| HepG2 | Cytotoxicity assay | Cytotoxicity against human HepG2 cells by MTT assay, IC50 = 32.6 μM. | 28371676 | |||
| A549 | Cytotoxicity assay | Cytotoxicity against human A549 cells by MTT assay, IC50 = 38.2 μM. | 28371676 | |||
| MCF7 | Antiproliferative assay | 48 to 72 hrs | Antiproliferative activity against human MCF7 cells incubated for 48 to 72 hrs by MTT assay, IC50 = 7.67 μM. | 28395218 | ||
| A549 | Antiproliferative assay | 48 to 72 hrs | Antiproliferative activity against human A549 cells incubated for 48 to 72 hrs by MTT assay, IC50 = 10.65 μM. | 28395218 | ||
| SGC7901 | Antiproliferative assay | 48 to 72 hrs | Antiproliferative activity against human SGC7901 cells incubated for 48 to 72 hrs by MTT assay, IC50 = 13.25 μM. | 28395218 | ||
| HepG2 | Antiproliferative assay | 48 to 72 hrs | Antiproliferative activity against human HepG2 cells incubated for 48 to 72 hrs by MTT assay, IC50 = 17.44 μM. | 28395218 | ||
| PC3 | Antiproliferative assay | 48 to 72 hrs | Antiproliferative activity against human PC3 cells incubated for 48 to 72 hrs by MTT assay, IC50 = 38.61 μM. | 28395218 | ||
| MCF7 | Growth inhibition assay | 48 hrs | Growth inhibition of human MCF7 cells after 48 hrs by SRB assay, GI50 = 1.67 μM. | 28400235 | ||
| A549 | Growth inhibition assay | 48 hrs | Growth inhibition of human A549 cells after 48 hrs by SRB assay, GI50 = 2.09 μM. | 28400235 | ||
| DU145 | Growth inhibition assay | 48 hrs | Growth inhibition of human DU145 cells after 48 hrs by SRB assay, GI50 = 5.82 μM. | 28400235 | ||
| T47D | Antiproliferative assay | 48 hrs | Antiproliferative activity against human T47D cells after 48 hrs by MTT assay, IC50 = 12.1 μM. | 28400238 | ||
| A549 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human A549 cells after 48 hrs by MTT assay, IC50 = 15.1 μM. | 28400238 | ||
| HepG2 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HepG2 cells after 48 hrs by MTT assay, IC50 = 20.1 μM. | 28400238 | ||
| HCT116 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HCT116 cells after 48 hrs by MTT assay, IC50 = 26.2 μM. | 28400238 | ||
| MCF7 | Cytotoxicity assay | Cytotoxicity against human MCF7 cells, IC50 = 7.9 μM. | 28431341 | |||
| HepG2 | Cytotoxicity assay | Cytotoxicity against human HepG2 cells, IC50 = 8.1 μM. | 28431341 | |||
| HCT116 | Cytotoxicity assay | Cytotoxicity against human HCT116 cells, IC50 = 9.9 μM. | 28431341 | |||
| PC3 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human PC3 cells after 48 hrs by MTT assay, IC50 = 1.2 μM. | 28431881 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells after 48 hrs by MTT assay, IC50 = 1.6 μM. | 28431881 | ||
| HeLa | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HeLa cells after 48 hrs by MTT assay, IC50 = 1.86 μM. | 28431881 | ||
| DU145 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human DU145 cells after 48 hrs by MTT assay, IC50 = 2.18 μM. | 28431881 | ||
| MDA-MB-231 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MDA-MB-231 cells after 48 hrs by MTT assay, IC50 = 4.33 μM. | 28431881 | ||
| HGC27 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HGC27 cells after 48 hrs by MTT assay, IC50 = 14.72 μM. | 28431881 | ||
| MGC803 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MGC803 cells after 72 hrs by MTT assay, IC50 = 6.56 μM. | 28456031 | ||
| HGC27 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HGC27 cells after 72 hrs by MTT assay, IC50 = 8.22 μM. | 28456031 | ||
| NCI-H1650 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human NCI-H1650 cells after 72 hrs by MTT assay, IC50 = 12.52 μM. | 28456031 | ||
| HCT116 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT116 cells after 72 hrs by MTT assay, IC50 = 4.16 μM. | 28458133 | ||
| PC3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human PC3 cells after 72 hrs by MTT assay, IC50 = 24.33 μM. | 28458133 | ||
| MCF7 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MCF7 cells after 72 hrs by MTT assay, IC50 = 28.73 μM. | 28458133 | ||
| HepG2 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HepG2 cells after 72 hrs by MTT assay, IC50 = 29.07 μM. | 28458133 | ||
| Caco2 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human Caco2 cells assessed as cell viability after 24 hrs by MTT assay, IC50 = 31 μM. | 28463785 | ||
| HeLa | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HeLa cells assessed as reduction in cell viability after 72 hrs by WST8 assay, IC50 = 13.9 μM. | 28488862 | ||
| HT-29 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HT-29 cells after 72 hrs by MTT assay, IC50 = 4.9 μM. | 28494251 | ||
| PC3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human PC3 cells after 72 hrs by MTT assay, IC50 = 24.3 μM. | 28494251 | ||
| HepG2 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HepG2 cells after 72 hrs by MTT assay, IC50 = 29.1 μM. | 28494251 | ||
| SW620 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SW620 cells after 72 hrs by MTT assay, IC50 = 0.08 μM. | 28525845 | ||
| CFPAC-1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human CFPAC-1 cells after 72 hrs by MTT assay, IC50 = 0.14 μM. | 28525845 | ||
| WI38 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human WI38 cells after 72 hrs by MTT assay, IC50 = 0.94 μM. | 28525845 | ||
| HeLa | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HeLa cells after 72 hrs by MTT assay, IC50 = 8.81 μM. | 28525845 | ||
| HepG2 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HepG2 cells after 72 hrs by MTT assay, IC50 = 8.9 μM. | 28525845 | ||
| HCT116 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HCT116 cells assessed as reduction in cell viability after 48 hrs by MTT assay, IC50 = 41.9 μM. | 28532669 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells assessed as decrease in cell viability after 72 hrs by WST8 assay, IC50 = 4.7 μM. | 28558970 | ||
| HeLa | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HeLa cells assessed as decrease in cell viability after 72 hrs by WST8 assay, IC50 = 5.3 μM. | 28558970 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells assessed as decrease in cell viability after 72 hrs by WST8 assay, IC50 = 5.6 μM. | 28558970 | ||
| WI38 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human WI38 cells assessed as decrease in cell viability after 72 hrs by WST8 assay, IC50 = 6.8 μM. | 28558970 | ||
| HCT116 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HCT116 cells assessed as decrease in cell viability after 48 hrs by MTT assay, IC50 = 4 μM. | 28623814 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MCF7 cells assessed as decrease in cell viability after 48 hrs by MTT assay, IC50 = 4.3 μM. | 28623814 | ||
| HepG2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 cells assessed as decrease in cell viability after 48 hrs by MTT assay, IC50 = 5.3 μM. | 28623814 | ||
| W138 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human W138 cells assessed as decrease in cell viability after 48 hrs by MTT assay, IC50 = 5.73 μM. | 28623814 | ||
| P388 | Cytotoxicity assay | 72 hrs | Cytotoxicity against mouse P388 cells incubated for 72 hrs by alamar blue assay, IC50 = 5.8 μM. | 28648460 | ||
| MOLT4 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MOLT4 cells incubated for 72 hrs by alamar blue assay, IC50 = 6.2 μM. | 28648460 | ||
| HT-29 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HT-29 cells incubated for 72 hrs by alamar blue assay, IC50 = 7.2 μM. | 28648460 | ||
| K562 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human K562 cells incubated for 72 hrs by alamar blue assay, IC50 = 30.5 μM. | 28648460 | ||
| MCF7 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MCF7 cells after 48 hrs by MTT assay, IC50 = 3.18 μM. | 28666860 | ||
| LNCAP | Antiproliferative assay | 48 hrs | Antiproliferative activity against human LNCAP cells after 48 hrs by MTT assay, IC50 = 5.21 μM. | 28666860 | ||
| PC3 | Cytotoxicity assay | Cytotoxic activity against human PC3 cells, IC50 = 1.6 μM. | 28697923 | |||
| HL60 | Cytotoxicity assay | Cytotoxic activity against human HL60 cells, IC50 = 2.8 μM. | 28697923 | |||
| HCT116 | Cytotoxicity assay | Cytotoxic activity against human HCT116 cells, IC50 = 17.01 μM. | 28697923 | |||
| HCT116 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HCT116 cells after 48 hrs by sulforhodamine B assay, GI50 = 5.47 μM. | 28716495 | ||
| CRL1790 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human CRL1790 cells assessed as reduction in cell viability after 48 hrs by sulforhodamine B assay, GI50 = 10.3 μM. | 28716495 | ||
| SW1573 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human SW1573 cells after 48 hrs by SRB assay, GI50 = 4.3 μM. | 28728108 | ||
| HBL100 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HBL100 cells after 48 hrs by SRB assay, GI50 = 5.5 μM. | 28728108 | ||
| hTERT-BJ | Antiproliferative assay | 48 hrs | Antiproliferative activity against human hTERT-BJ cells after 48 hrs by SRB assay, GI50 = 5.5 μM. | 28728108 | ||
| HeLa | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HeLa cells after 48 hrs by SRB assay, GI50 = 15 μM. | 28728108 | ||
| T47D | Antiproliferative assay | 48 hrs | Antiproliferative activity against human T47D cells after 48 hrs by SRB assay, GI50 = 47 μM. | 28728108 | ||
| WiDr | Antiproliferative assay | 48 hrs | Antiproliferative activity against human WiDr cells after 48 hrs by SRB assay, GI50 = 49 μM. | 28728108 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity in human A549 cells assessed as reduction in cell viability incubated for 48 hrs by MTT assay, IC50 = 34.47 μM. | 28754470 | ||
| T24 | Cytotoxicity assay | 48 hrs | Cytotoxicity in human T24 cells assessed as reduction in cell viability incubated for 48 hrs by MTT assay, IC50 = 40.14 μM. | 28754470 | ||
| SPCA2 | Cytotoxicity assay | 48 hrs | Cytotoxicity in human SPCA2 cells assessed as reduction in cell viability incubated for 48 hrs by MTT assay, IC50 = 44.34 μM. | 28754470 | ||
| HepG2 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HepG2 cells after 48 hrs by MTT assay, IC50 = 28.8 μM. | 28756264 | ||
| SKOV3 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human SKOV3 cells after 48 hrs by MTT assay, IC50 = 32.5 μM. | 28756264 | ||
| NCI-H460 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human NCI-H460 cells after 48 hrs by MTT assay, IC50 = 36 μM. | 28756264 | ||
| MCF7 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MCF7 cells after 48 hrs by MTT assay, IC50 = 38.5 μM. | 28756264 | ||
| HL-7702 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HL-7702 cells after 48 hrs by MTT assay, IC50 = 46 μM. | 28756264 | ||
| EC109 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human EC109 cells after 72 hrs by MTT assay, IC50 = 5.01 μM. | 28759876 | ||
| MGC803 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MGC803 cells after 72 hrs by MTT assay, IC50 = 6.89 μM. | 28759876 | ||
| MCF7 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MCF7 cells after 72 hrs by MTT assay, IC50 = 7.21 μM. | 28759876 | ||
| GES-1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human GES-1 cells assessed as reduction in cell viability after 72 hrs by MTT assay, IC50 = 8.59 μM. | 28759876 | ||
| HGC27 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HGC27 cells after 72 hrs by MTT assay, IC50 = 11.02 μM. | 28759876 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells assessed as reduction in cell viability after 72 hrs by MTT assay, IC50 = 5.2 μM. | 28759877 | ||
| PC3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human PC3 cells assessed as reduction in cell viability after 72 hrs by MTT assay, IC50 = 13.7 μM. | 28759877 | ||
| SKOV3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human SKOV3 cells assessed as reduction in cell viability after 72 hrs by MTT assay, IC50 = 20.8 μM. | 28759877 | ||
| MDA-MB-231 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MDA-MB-231 cells assessed as reduction in cell viability after 72 hrs by MTT assay, IC50 = 24.5 μM. | 28759877 | ||
| MGC803 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MGC803 cells after 48 hrs by MTT assay, IC50 = 9.79 μM. | 28763643 | ||
| PC3 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human PC3 cells after 48 hrs by MTT assay, IC50 = 12.87 μM. | 28763643 | ||
| SH-SY5Y | Antiproliferative assay | 48 hrs | Antiproliferative activity against human SH-SY5Y cells after 48 hrs by MTT assay, GI50 = 12 μM. | 28763647 | ||
| K562 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human K562 cells after 48 hrs by MTT assay, GI50 = 19 μM. | 28763647 | ||
| AGS | Antiproliferative assay | 48 hrs | Antiproliferative activity against human AGS cells after 48 hrs by MTT assay, GI50 = 25 μM. | 28763647 | ||
| HeLa | Cytotoxicity assay | 24 hrs | Cytotoxicity against human HeLa cells assessed as reduction in cell viability after 24 hrs by MTT assay, IC50 = 2.9 μM. | 28838694 | ||
| EC109 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human EC109 cells after 48 hrs by MTT assay, IC50 = 6.21 μM. | 28838695 | ||
| MGC803 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MGC803 cells after 48 hrs by MTT assay, IC50 = 7.52 μM. | 28838695 | ||
| MKN45 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MKN45 cells after 48 hrs by MTT assay, IC50 = 8.89 μM. | 28838695 | ||
| NCI-H1650 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human NCI-H1650 cells after 48 hrs by MTT assay, IC50 = 14.25 μM. | 28838695 | ||
| EC109 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human EC109 cells after 48 hrs by MTT assay, IC50 = 6.21 μM. | 28863355 | ||
| MGC803 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MGC803 cells after 48 hrs by MTT assay, IC50 = 7.52 μM. | 28863355 | ||
| MKN45 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MKN45 cells after 48 hrs by MTT assay, IC50 = 8.89 μM. | 28863355 | ||
| GES-1 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human GES-1 cells after 48 hrs by MTT assay, IC50 = 9.02 μM. | 28863355 | ||
| A549 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human A549 cells after 48 hrs by MTT assay, IC50 = 12.92 μM. | 28863355 | ||
| NCI-H1650 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human NCI-H1650 cells after 48 hrs by MTT assay, IC50 = 14.25 μM. | 28863355 | ||
| A549 | Growth inhibition assay | 48 hrs | Growth inhibition of human A549 cells after 48 hrs by SRB assay, GI50 = 4.2 μM. | 28898082 | ||
| PC3 | Growth inhibition assay | 48 hrs | Growth inhibition of human PC3 cells after 48 hrs by SRB assay, GI50 = 11.7 μM. | 28898082 | ||
| PC3 | Cytotoxicity assay | 48 hrs | Cytotoxicity in human PC3 cells incubated for 48 hrs by MTT assay, IC50 = 1.2 μM. | 28923381 | ||
| DU145 | Cytotoxicity assay | 48 hrs | Cytotoxicity in human DU145 cells incubated for 48 hrs by MTT assay, IC50 = 2.18 μM. | 28923381 | ||
| HCT116 | Cytotoxicity assay | 48 hrs | Cytotoxicity in human HCT116 cells incubated for 48 hrs by MTT assay, IC50 = 8.62 μM. | 28923381 | ||
| HT-29 | Antitumor assay | 48 hrs | Antitumor activity against human HT-29 cells assessed as inhibition of cell viability incubated for 48 hrs by MTT assay, IC50 = 4.6 μM. | 28926764 | ||
| MCF7 | Antitumor assay | 48 hrs | Antitumor activity against human MCF7 cells assessed as inhibition of cell viability incubated for 48 hrs by MTT assay, IC50 = 5.7 μM. | 28926764 | ||
| WI38 | Cytotoxicity assay | 48 hrs | Cytotoxicity in human WI38 cells assessed as inhibition of cell viability incubated for 48 hrs by MTT assay, IC50 = 5.7 μM. | 28926764 | ||
| HepG2 | Antitumor assay | 48 hrs | Antitumor activity against human HepG2 cells assessed as inhibition of cell viability incubated for 48 hrs by MTT assay, IC50 = 6.9 μM. | 28926764 | ||
| L02 | Antiproliferative assay | 48 hrs | Anti-proliferative activity against human L02 cells after 48 hrs by MTT assay, IC50 = 19.1 μM. | 28947149 | ||
| AGS | Antiproliferative assay | 48 hrs | Anti-proliferative activity against human AGS cells after 48 hrs by MTT assay, IC50 = 29.6 μM. | 28947149 | ||
| B16 | Cytotoxicity assay | 72 hrs | Cytotoxicity in mouse B16 cells assessed as reduction in cell viability incubated for 72 hrs by MTT assay, IC50 = 1.19 μM. | 28987604 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity in human A549 cells assessed as reduction in cell viability incubated for 72 hrs by MTT assay, IC50 = 8.9 μM. | 28987604 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity in human HepG2 cells assessed as reduction in cell viability incubated for 72 hrs by MTT assay, IC50 = 10.4 μM. | 28987604 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity in human MCF7 cells assessed as reduction in cell viability incubated for 72 hrs by MTT assay, IC50 = 14.7 μM. | 28987604 | ||
| HL60 | Antiproliferative assay | 24 hrs | Antiproliferative activity against human HL60 cells treated for 24 hrs measured after 72 hrs by MTT assay, IC50 = 3.69 μM. | 29037951 | ||
| THP1 | Antiproliferative assay | 24 hrs | Antiproliferative activity against human THP1 cells treated for 24 hrs measured after 72 hrs by MTT assay, IC50 = 4.87 μM. | 29037951 | ||
| PC3 | Antiproliferative assay | 24 hrs | Antiproliferative activity against human PC3 cells treated for 24 hrs measured after 72 hrs by MTT assay, IC50 = 23.94 μM. | 29037951 | ||
| HepG2 | Antiproliferative assay | 24 hrs | Antiproliferative activity against human HepG2 cells treated for 24 hrs measured after 72 hrs by MTT assay, IC50 = 30.48 μM. | 29037951 | ||
| SH-SY5Y | Antiproliferative assay | 48 hrs | Antiproliferative activity against human SH-SY5Y cells after 48 hrs by MTT assay, GI50 = 11.9 μM. | 29040954 | ||
| K562 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human K562 cells after 48 hrs by MTT assay, GI50 = 18.5 μM. | 29040954 | ||
| AGS | Antiproliferative assay | 48 hrs | Antiproliferative activity against human AGS cells after 48 hrs by MTT assay, GI50 = 24.6 μM. | 29040954 | ||
| EC109 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human EC109 cells assessed as decrease in cell viability after 48 hrs by MTT assay, IC50 = 6.21 μM. | 29113745 | ||
| MGC803 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MGC803 cells assessed as decrease in cell viability after 48 hrs by MTT assay, IC50 = 7.52 μM. | 29113745 | ||
| MKN45 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MKN45 cells assessed as decrease in cell viability after 48 hrs by MTT assay, IC50 = 8.89 μM. | 29113745 | ||
| NCI-H1650 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human NCI-H1650 cells assessed as decrease in cell viability after 48 hrs by MTT assay, IC50 = 14.25 μM. | 29113745 | ||
| HL60 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HL60 cells after 72 hrs by tryphan blue staining based hematocytometric method, IC50 = 2.8 μM. | 29131616 | ||
| PC3 | Antiproliferative assay | Antiproliferative activity against human PC3 cells by MTT assay, IC50 = 22.02 μM. | 29131616 | |||
| EAC | Cytotoxicity assay | 48 hrs | Cytotoxicity against mouse EAC cells assessed as reduction in cell viability after 48 hrs by MTT assay, IC50 = 9.2 μM. | 29133037 | ||
| EAC | Cytotoxicity assay | 48 hrs | Cytotoxicity against mouse EAC cells assessed as reduction in cell viability after 48 hrs by trypan blue exclusion assay, IC50 = 9.6 μM. | 29133037 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MCF7 cells assessed as reduction in cell viability after 48 hrs by MTT assay, IC50 = 10.2 μM. | 29133037 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MCF7 cells assessed as reduction in cell viability after 48 hrs by trypan blue exclusion assay, IC50 = 10.6 μM. | 29133037 | ||
| DLA | Cytotoxicity assay | 48 hrs | Cytotoxicity against mouse DLA cells assessed as reduction in cell viability after 48 hrs by trypan blue exclusion assay, IC50 = 10.6 μM. | 29133037 | ||
| DLA | Cytotoxicity assay | 48 hrs | Cytotoxicity against mouse DLA cells assessed as reduction in cell viability after 48 hrs by MTT assay, IC50 = 10.8 μM. | 29133037 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells assessed as reduction in cell viability after 48 hrs by trypan blue exclusion assay, IC50 = 11.2 μM. | 29133037 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells assessed as reduction in cell viability after 48 hrs by MTT assay, IC50 = 11.4 μM. | 29133037 | ||
| MDA-MB-468 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MDA-MB-468 cells assessed as reduction in cell viability after 48 hrs by trypan blue exclusion assay, IC50 = 44.6 μM. | 29133037 | ||
| MDA-MB-468 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MDA-MB-468 cells assessed as reduction in cell viability after 48 hrs by MTT assay, IC50 = 44.9 μM. | 29133037 | ||
| SW620 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SW620 cells after 72 hrs by MTT assay, IC50 = 0.08 μM. | 29133046 | ||
| CFPAC-1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human CFPAC-1 cells after 72 hrs by MTT assay, IC50 = 0.14 μM. | 29133046 | ||
| WI38 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human WI38 cells after 72 hrs by MTT assay, IC50 = 0.94 μM. | 29133046 | ||
| A549 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A549 cells after 72 hrs by MTT assay, IC50 = 2.8 μM. | 29133046 | ||
| HeLa | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HeLa cells after 72 hrs by MTT assay, IC50 = 8.81 μM. | 29133046 | ||
| K562 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human K562 cells assessed as reduction in cell viability after 48 hrs by MTT assay, IC50 = 9.49 μM. | 29133049 | ||
| WM9 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human WM9 cells assessed as reduction in cell viability after 48 hrs by MTT assay, IC50 = 15.8 μM. | 29133049 | ||
| HEL | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HEL cells assessed as reduction in cell viability after 48 hrs by MTT assay, IC50 = 15.98 μM. | 29133049 | ||
| MDA-MB-231 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MDA-MB-231 cells assessed as reduction in cell viability after 48 hrs by MTT assay, IC50 = 17.74 μM. | 29133049 | ||
| PC3 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human PC3 cells assessed as reduction in cell viability after 48 hrs by MTT assay, IC50 = 19.87 μM. | 29133049 | ||
| PC3 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human PC3 cells after 48 hrs by MTT assay, IC50 = 4.75 μM. | 29133051 | ||
| A549 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human A549 cells after 48 hrs by MTT assay, IC50 = 8.48 μM. | 29133051 | ||
| SGC7901 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human SGC7901 cells after 48 hrs by MTT assay, IC50 = 10.98 μM. | 29133051 | ||
| MGC803 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MGC803 cells after 48 hrs by MTT assay, IC50 = 14.15 μM. | 29133051 | ||
| HT-29 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HT-29 cells assessed as cell growth inhibition after 48 hrs by MTT assay, IC50 = 8.3 μM. | 29133061 | ||
| HCT15 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HCT15 cells after 48 hrs by MTT assay, IC50 = 7.53 μM. | 29174813 | ||
| Bel7402 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human Bel7402 cells after 48 hrs by MTT assay, IC50 = 20.93 μM. | 29174813 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MCF7 cells after 48 hrs by MTT assay, IC50 = 28.97 μM. | 29174813 | ||
| U937 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human U937 cells in presence of ubenimex after 48 hrs by MTT assay, IC50 = 9.56 μM. | 29202398 | ||
| HepG2 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HepG2 cells in presence of ubenimex after 48 hrs by MTT assay, IC50 = 14.52 μM. | 29202398 | ||
| A549 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human A549 cells in presence of ubenimex after 48 hrs by MTT assay, IC50 = 15.93 μM. | 29202398 | ||
| A549 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human A549 cells after 48 hrs by MTT assay, IC50 = 17.02 μM. | 29202398 | ||
| H7402 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human H7402 cells in presence of ubenimex after 48 hrs by MTT assay, IC50 = 17.58 μM. | 29202398 | ||
| HepG2 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HepG2 cells after 48 hrs by MTT assay, IC50 = 18.35 μM. | 29202398 | ||
| H7402 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human H7402 cells after 48 hrs by MTT assay, IC50 = 21.87 μM. | 29202398 | ||
| PLC/PRF/5 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human PLC/PRF/5 cells in presence of ubenimex after 48 hrs by MTT assay, IC50 = 22.81 μM. | 29202398 | ||
| U937 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human U937 cells after 48 hrs by MTT assay, IC50 = 28.76 μM. | 29202398 | ||
| PLC/PRF/5 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human PLC/PRF/5 cells after 48 hrs by MTT assay, IC50 = 35.66 μM. | 29202398 | ||
| ES2 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human ES2 cells in presence of ubenimex after 48 hrs by MTT assay, IC50 = 37.05 μM. | 29202398 | ||
| U266 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human U266 cells in presence of ubenimex after 48 hrs by MTT assay, IC50 = 43.48 μM. | 29202398 | ||
| HCT116 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HCT116 cells in presence of ubenimex after 48 hrs by MTT assay, IC50 = 44.06 μM. | 29202398 | ||
| MCF7 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MCF7 cells after 72 hrs by MTT assay, IC50 = 2.35 μM. | 29223098 | ||
| HCT116 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HCT116 cells after 48 hrs by MTT assay, IC50 = 8.638 μM. | 29223717 | ||
| 143B | Antiproliferative assay | 48 hrs | Antiproliferative activity against human 143B cells after 48 hrs by MTT assay, IC50 = 25.216 μM. | 29223717 | ||
| MCF7 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MCF7 cells after 48 hrs by MTT assay, IC50 = 32.626 μM. | 29223717 | ||
| HCT116 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT116 cells after 72 hrs by MTT assay, IC50 = 29.5 μM. | 29232583 | ||
| SGC7901 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SGC7901 cells after 72 hrs by MTT assay, IC50 = 34.4 μM. | 29232583 | ||
| SMMC7721 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SMMC7721 cells after 72 hrs by MTT assay, IC50 = 37.8 μM. | 29232583 | ||
| HuH7 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HuH7 cells after 72 hrs by MTT assay, IC50 = 5.63 μM. | 29316536 | ||
| HCT116 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT116 cells after 72 hrs by MTT assay, IC50 = 11.29 μM. | 29316536 | ||
| MGC803 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MGC803 cells after 72 hrs by MTT assay, IC50 = 25.54 μM. | 29316536 | ||
| MGC803 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MGC803 cells after 72 hrs by MTT assay, IC50 = 7.86 μM. | 29407946 | ||
| NCI-H1650 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human NCI-H1650 cells after 72 hrs by MTT assay, IC50 = 14.63 μM. | 29407946 | ||
| PC3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human PC3 cells after 72 hrs by MTT assay, IC50 = 27.61 μM. | 29407946 | ||
| HL60 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HL60 cells after 72 hrs by MTT assay, IC50 = 2.37 μM. | 29407983 | ||
| Bel7402 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human Bel7402 cells after 72 hrs by MTT assay, IC50 = 18.49 μM. | 29407983 | ||
| PC3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human PC3 cells after 72 hrs by MTT assay, IC50 = 23.86 μM. | 29407983 | ||
| EC9706 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human EC9706 cells after 72 hrs by MTT assay, IC50 = 5.13 μM. | 29475156 | ||
| EC109 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human EC109 cells after 72 hrs by MTT assay, IC50 = 6.02 μM. | 29475156 | ||
| PC3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human PC3 cells after 72 hrs by MTT assay, IC50 = 6.27 μM. | 29475156 | ||
| MGC803 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MGC803 cells after 72 hrs by MTT assay, IC50 = 9.24 μM. | 29475156 | ||
| SMMC7721 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SMMC7721 cells after 72 hrs by MTT assay, IC50 = 13.7 μM. | 29475156 | ||
| MCF7 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MCF7 cells after 72 hrs by MTT assay, IC50 = 18.75 μM. | 29475156 | ||
| MCF7 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MCF7 cells after 48 hrs by MTT assay, IC50 = 15.78 μM. | 29486954 | ||
| HCT116 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HCT116 cells after 48 hrs by MTT assay, IC50 = 17.25 μM. | 29486954 | ||
| A549 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human A549 cells after 48 hrs by MTT assay, IC50 = 19.52 μM. | 29486954 | ||
| HepG2 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HepG2 cells after 48 hrs by MTT assay, IC50 = 23.6 μM. | 29486954 | ||
| HeLa | Cytotoxicity assay | Cytotoxicity against human HeLa cells assessed as decrease in cell viability by MTT assay, IC50 = 28 μM. | 29489361 | |||
| A549 | Cytotoxicity assay | Cytotoxicity against human A549 cells assessed as decrease in cell viability by MTT assay, IC50 = 36.8 μM. | 29489361 | |||
| QSG7701 | Antiproliferative assay | 24 hrs | Antiproliferative activity against human QSG7701 cells after 24 hrs by CCK-8 assay, IC50 = 6.92 μM. | 29499490 | ||
| SMMC7721 | Antiproliferative assay | 24 hrs | Antiproliferative activity against human SMMC7721 cells after 24 hrs by CCK-8 assay, IC50 = 12.47 μM. | 29499490 | ||
| QGY7703 | Antiproliferative assay | 24 hrs | Antiproliferative activity against human QGY7703 cells after 24 hrs by CCK-8 assay, IC50 = 14.2 μM. | 29499490 | ||
| HepG2 | Antiproliferative assay | 24 hrs | Antiproliferative activity against human HepG2 cells after 24 hrs by CCK-8 assay, IC50 = 15.74 μM. | 29499490 | ||
| HL60 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HL60 cells after 72 hrs by MTT assay, IC50 = 2.37 μM. | 29524728 | ||
| Bel7402 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human Bel7402 cells after 72 hrs by MTT assay, IC50 = 18.49 μM. | 29524728 | ||
| PC3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human PC3 cells after 72 hrs by MTT assay, IC50 = 23.86 μM. | 29524728 | ||
| MGC803 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MGC803 cells after 72 hrs by MTT assay, IC50 = 8.66 μM. | 29635165 | ||
| PC3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human PC3 cells after 72 hrs by MTT assay, IC50 = 12.33 μM. | 29635165 | ||
| GES-1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human GES-1 cells after 72 hrs by MTT assay, IC50 = 12.51 μM. | 29635165 | ||
| A549 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A549 cells after 72 hrs by MTT assay, IC50 = 13.18 μM. | 29635165 | ||
| TE1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human TE1 cells after 72 hrs by MTT assay, IC50 = 16.53 μM. | 29635165 | ||
| HL60 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HL60 cells after 72 hrs by MTT assay, IC50 = 3.46 μM. | 29635169 | ||
| Bel7402 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human Bel7402 cells after 72 hrs by MTT assay, IC50 = 18.97 μM. | 29635169 | ||
| SGC7901 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SGC7901 cells after 72 hrs by MTT assay, IC50 = 19.84 μM. | 29635169 | ||
| MCF7 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MCF7 cells after 72 hrs by MTT assay, IC50 = 26.91 μM. | 29635169 | ||
| Caco2 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human Caco2 cells after 72 hrs by MTT assay, IC50 = 38.77 μM. | 29635169 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity in human MCF7 cells assessed as reduction in cell viability incubated for 48 hrs by MTT assay, IC50 = 16.7 μM. | 29657100 | ||
| MDA-MB-231 | Cytotoxicity assay | 48 hrs | Cytotoxicity in human MDA-MB-231 cells assessed as reduction in cell viability incubated for 48 hrs by MTT assay, IC50 = 35.91 μM. | 29657100 | ||
| DU145 | Cytotoxicity assay | 48 hrs | Cytotoxicity in human DU145 cells assessed as reduction in cell viability incubated for 48 hrs by MTT assay, IC50 = 39.17 μM. | 29657100 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity in human A549 cells assessed as reduction in cell viability incubated for 48 hrs by MTT assay, IC50 = 39.86 μM. | 29657100 | ||
| HepG2 | Cytotoxicity assay | 6 days | Cytotoxicity against human HepG2 cells after 6 days by MTT assay, IC50 = 12 μM. | 29786436 | ||
| SGC7901 | Cytotoxicity assay | 6 days | Cytotoxicity against human SGC7901 cells after 6 days by MTT assay, IC50 = 12 μM. | 29786436 | ||
| HepG2 | Antiproliferative assay | Antiproliferative activity against human HepG2 cells by MTT assay, IC50 = 1.7 μM. | 29798826 | |||
| HeLa | Antiproliferative assay | Antiproliferative activity against human HeLa cells by MTT assay, IC50 = 1.8 μM. | 29798826 | |||
| MCF7 | Antiproliferative assay | Antiproliferative activity against human MCF7 cells by MTT assay, IC50 = 1.8 μM. | 29798826 | |||
| COLO205 | Antiproliferative assay | Antiproliferative activity against human COLO205 cells by MTT assay, IC50 = 1.9 μM. | 29798826 | |||
| HEK293 | Antiproliferative assay | Antiproliferative activity against human HEK293 cells by MTT assay, IC50 = 19.6 μM. | 29798826 | |||
| A549 | Cytotoxicity assay | Cytotoxicity against human A549 cells by MTS assay, IC50 = 3.52 μM. | 29843103 | |||
| HCT116 | Cytotoxicity assay | Cytotoxicity against human HCT116 cells by MTS assay, IC50 = 5.53 μM. | 29843103 | |||
| HeLa | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HeLa cells assessed as decrease in cell viability after 72 hrs by MTT assay, IC50 = 7 μM. | 29908443 | ||
| McCoy | Cytotoxicity assay | 72 hrs | Cytotoxicity against mouse McCoy cells assessed as decrease in cell viability after 72 hrs by MTT assay, IC50 = 15.7 μM. | 29908443 | ||
| PC3 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human PC3 cells after 48 hrs by MTT assay, IC50 = 8.45 μM. | 29958763 | ||
| HT-29 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HT-29 cells after 48 hrs by MTT assay, IC50 = 22.23 μM. | 29958763 | ||
| HeLa | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HeLa cells after 48 hrs by MTT assay, IC50 = 30.21 μM. | 30007564 | ||
| HepG2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 cells after 48 hrs by MTT assay, IC50 = 36.62 μM. | 30007564 | ||
| 2008 | Cytotoxicity assay | Cytotoxicity against human 2008 cells, IC50 = 6 μM. | 30035541 | |||
| IGROV1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human IGROV1 cells after 72 hrs, IC50 = 10.6 μM. | 30035541 | ||
| C13 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human C13 cells after 72 hrs, IC50 = 12.1 μM. | 30035541 | ||
| HCT8 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT8 cells after 72 hrs by MTT assay, IC50 = 28.9 μM. | 30037494 | ||
| DLD1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human DLD1 cells after 72 hrs by MTT assay, IC50 = 33 μM. | 30037494 | ||
| CCD-841-CoN | Antiproliferative assay | 72 hrs | Antiproliferative activity against human CCD-841-CoN cells after 72 hrs by MTT assay, IC50 = 35.7 μM. | 30037494 | ||
| SKOV3 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human SKOV3 cells after 48 hrs by MTT assay, IC50 = 26.34 μM. | 30092368 | ||
| HepG2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 cells after 48 hrs by MTT assay, IC50 = 31.98 μM. | 30092368 | ||
| Bel7404 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human Bel7404 cells after 48 hrs by MTT assay, IC50 = 40.21 μM. | 30092368 | ||
| NCI-H460 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human NCI-H460 cells after 48 hrs by MTT assay, IC50 = 45.44 μM. | 30092368 | ||
| RKO | Growth inhibition assay | 48 hrs | Growth inhibition of human RKO cells after 48 hrs by CCK-8 assay, IC50 = 10.2 μM. | 30106289 | ||
| LoVo | Growth inhibition assay | 48 hrs | Growth inhibition of human LoVo cells after 48 hrs by CCK-8 assay, IC50 = 11.5 μM. | 30106289 | ||
| MCF7 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MCF7 cells after 72 hrs by MTT assay, IC50 = 26.14 μM. | 30142612 | ||
| MGC803 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MGC803 cells after 72 hrs by MTT assay, IC50 = 31.04 μM. | 30142612 | ||
| HCT116 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT116 cells after 72 hrs by MTT assay, IC50 = 38.3 μM. | 30142612 | ||
| HepG2 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HepG2 cells after 72 hrs by MTT assay, IC50 = 47.97 μM. | 30142612 | ||
| A549 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A549 cells after 72 hrs by MTT assay, IC50 = 2.05 μM. | 30243153 | ||
| Bel7402 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human Bel7402 cells after 72 hrs by MTT assay, IC50 = 18.65 μM. | 30243153 | ||
| MCF7 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MCF7 cells after 72 hrs by MTT assay, IC50 = 27.89 μM. | 30243153 | ||
| T84 | Antiproliferative assay | 3 days | Antiproliferative activity against human T84 cells after 3 days by sulforhodamine B assay, IC50 = 3.4 μM. | 30248657 | ||
| A549 | Antiproliferative assay | 3 days | Antiproliferative activity against human A549 cells after 3 days by sulforhodamine B assay, IC50 = 7.1 μM. | 30248657 | ||
| MCF7 | Antiproliferative assay | 3 days | Antiproliferative activity against human MCF7 cells after 3 days by sulforhodamine B assay, IC50 = 7.8 μM. | 30248657 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells after 72 hrs by MTT assay, IC50 = 4.3 μM. | 30318440 | ||
| HeLa | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HeLa cells after 72 hrs by MTT assay, IC50 = 10.75 μM. | 30318440 | ||
| HCT116 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT116 cells after 72 hrs by MTT assay, IC50 = 11.56 μM. | 30318440 | ||
| NCI-H460 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human NCI-H460 cells after 72 hrs by MTT assay, IC50 = 11.75 μM. | 30318440 | ||
| HCT15 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT15 cells after 72 hrs by MTT assay, IC50 = 11.87 μM. | 30318440 | ||
| MDA-MB-231 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MDA-MB-231 cells after 72 hrs by MTT assay, IC50 = 21.05 μM. | 30318440 | ||
| Click to View More Cell Line Experimental Data | ||||||
Mechanism of Action
| Targets |
|
|---|
In vitro |
||||
| In vitro | Adrucil is an analogue of uracil with a fluorine atom at the C-5 position in place of hydrogen. It rapidly enters the cell using the same facilitated transport mechanism as uracil. Adrucil is converted intracellularly to several active metabolites: fluorodeoxyuridine monophosphate (FdUMP), fluorodeoxyuridine triphosphate (FdUTP) and fluorouridine triphosphate (FUTP). The Adrucil metabolite FdUMP binds to the nucleotide-binding site of TS, forming a stable ternary complex with the enzyme and CH2THF, thereby blocking binding of the normal substrate dUMP and inhibiting dTMP synthesis. Metabolite of Adrucil also can be misincorporated into DNA, leading to DNA strand breaks and cell death. The pro-apoptosis effects of Adrucil may be related to its activation of tumor suppressor p53. Loss of p53 function reduces cellular sensitivity to Adrucil. [1] Adrucil is able to inhibit the survival and induce apoptosis of a board range of cancer cells. Adrucil suppresses viabilities of the nasopharyngeal carcinoma cell line CNE2 and HONE1 [2], pancreatic cancer cell lines Capan-1 [3], and human colon carcinoma cell line HT-29 [4] with IC50 of 9 μg/mL, 3 μg/mL, 0.22 μM, 2.5 μM, respectively. | |||
|---|---|---|---|---|
| Cell Research | Cell lines | Human colon carcinoma cell line HT-29 | ||
| Concentrations | ~25 μM | |||
| Incubation Time | 7 days | |||
| Method | Growth inhibition is measured after treatment of cells with Adrucil for 7 days in 96-well plates (4000 HT-29 cells/well in RPMI 1640 medium with 10% dialyzed fetal bovine serum); increasing concentrations of Adrucil are added after allowing for cell attachment overnight. At the end of incubation, cells are rinsed three times with phosphate-buffered saline (pH 7.4), fixed with 10% trichloroacetic acid for 60 min at 4 ℃, washed five times with deionized water, and stained with 0.4% sulforhoda-mine B solution for 15 min at room temperature. Unstained sulforhodamine B is removed by rinsing with 1% glacial acetic acid. Afterwards, stained cell proteins are dried and dissolved with 10 mM Tris-HCl. The optical density value is measured using a detector at 540 nm wavelength. | |||
| Experimental Result Images | Methods | Biomarkers | Images | PMID |
| Western blot | p53 / PUMA / c-PARP |
|
25965911 | |
| Immunofluorescence | non-phospho β-catenin β-catenin Sox2 / Oct4 / Nanog / ABCG2 p65 / p-p65(Ser536) / p53 / p-p53(Ser15) phospho-histone H3 caveolin-3 |
|
28588704 | |
| Growth inhibition assay | Cell proliferation Cell viability |
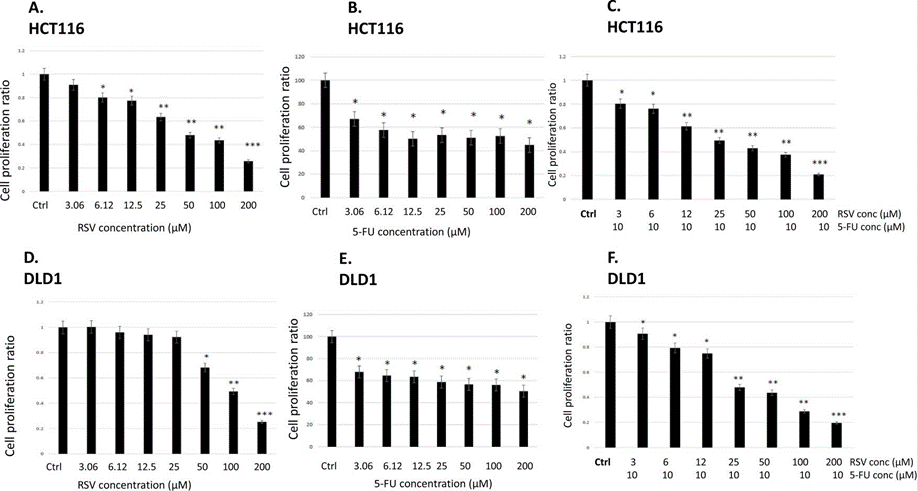
|
30250641 | |
In Vivo |
||
| In vivo | Adrucil is widely used in the treatment of a range of cancers, including colorectal and breast cancers. [1] 100mg/kg Adrucil significantly suppresses tumor growth of murine colon carcinomas Colon 38 with tumor-doubling time (TD), growth-delay factor (GDF), and T/C of 26.5 days, 4.4, and 14%. [5] | |
|---|---|---|
| Animal Research | Animal Models | Murine colon carcinomas Colon 38 |
| Dosages | 100 mg/kg | |
| Administration | i.p. weekly | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06361316 | Recruiting | Pancreatic Cancer|Adjuvant Therapy |
Kuirong Jiang|CSPC Ouyi Pharmaceutical Co. Ltd.|The First Affiliated Hospital with Nanjing Medical University |
April 8 2024 | Not Applicable |
| NCT05780684 | Recruiting | Colorectal Cancer|Esophagus Cancer|Appendix Cancer|Small Bowel Cancer|Ampullary Cancer |
Dartmouth-Hitchcock Medical Center |
July 14 2023 | Not Applicable |
| NCT05362955 | Active not recruiting | CIN 2/3|HIV Infections |
UNC Lineberger Comprehensive Cancer Center |
April 26 2023 | Phase 1 |
| NCT05620524 | Recruiting | TDM of 5-FU|Pharmacokinetic Observational Study |
Catharina Ziekenhuis Eindhoven |
December 19 2022 | -- |
| NCT05505539 | Withdrawn | Oral Leukoplakia |
University of California San Francisco |
September 30 2022 | Phase 1 |
References |
|
Chemical Information
| Molecular Weight | 130.08 | Formula | C4H3FN2O2 |
| CAS No. | 51-21-8 | SDF | Download SDF |
| Synonyms | Fluorouracil, NSC 19893 | ||
| Smiles | C1=C(C(=O)NC(=O)N1)F | ||
Storage and Stability
| Storage (From the date of receipt) | |||
|
In vitro |
DMSO : 26 mg/mL ( (199.87 mM) Moisture-absorbing DMSO reduces solubility. Please use fresh DMSO.) Water : 10 mg/mL Ethanol : Insoluble |
Molecular Weight Calculator |
|
In vivo Add solvents to the product individually and in order. |
In vivo Formulation Calculator |
|||||
Preparing Stock Solutions
Molarity Calculator
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Tech Support
Answers to questions you may have can be found in the inhibitor handling instructions. Topics include how to prepare stock solutions, how to store inhibitors, and issues that need special attention for cell-based assays and animal experiments.
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
* Indicates a Required Field
Frequently Asked Questions
Question 1:
I was wondering if the product #s1209 (5-fluorouracil) is suitable to inject into mice ?
Answer:
S1209 is suitable to inject (I.P.) into mice as indicating in this paper: http://www.ncbi.nlm.nih.gov/pubmed/8995503.