research use only
Ulipristal Acetate (CDB 2914) Estrogen/progestogen Receptor modulator
Cat.No.S3081
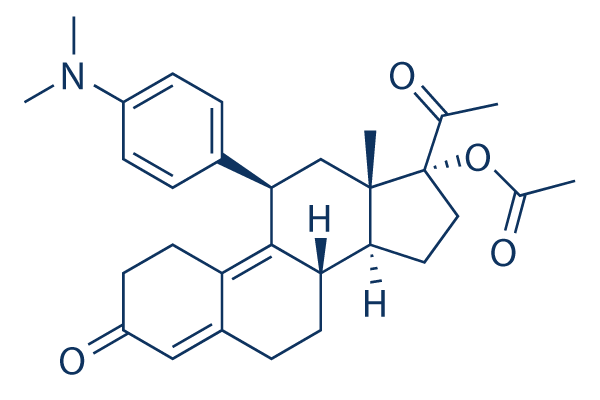
Chemical Structure
Molecular Weight: 475.62
Quality Control
| Related Targets | Adrenergic Receptor GPR Androgen Receptor Glucocorticoid Receptor ACE RAAS Progesterone Receptor Opioid Receptor PGES THR |
|---|---|
| Other Estrogen/progestogen Receptor Inhibitors | Elacestrant (RAD1901) Dihydrochloride MPP dihydrochloride Cholesterol Endoxifen HCl G15 Chrysin Licochalcone A AZD9496 PHTPP Liquiritigenin |
Solubility
|
In vitro |
DMSO
: 83 mg/mL
(174.5 mM)
Ethanol : 14 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 475.62 | Formula | C30H37NO4 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 126784-99-4 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | HRP 2000, RU 44675 | Smiles | CC(=O)C1(CCC2C1(CC(C3=C4CCC(=O)C=C4CCC23)C5=CC=C(C=C5)N(C)C)C)OC(=O)C | ||
Mechanism of Action
| Targets/IC50/Ki |
Progesterone receptor
|
|---|---|
| In vitro |
Ulipristal acetate (CDB 2914) has partial agonistic as well as antagonistic effects on the progesterone receptor. It also binds to the glucocorticoid receptor, but has no relevant affinity to the estrogen, androgen and mineralocorticoid receptors.
|
| In vivo |
Based on clinical trials, Ulipristal Acetate (CDB 2914) seems to be a reasonably tolerable and effective method of emergency contraception when used within 120 hours of intercourse. It is at least as effective as LNG when used within the first 72 hours after unprotected intercourse. However, this compound may be more effective than LNG when used between 72 to 120 hours after unprotected intercourse, extending the window of opportunity for emergency contraception.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT03349190 | Completed | Infertility Female|Fibroid Uterus |
ASSOCIATION POUR LE DEVELOPPEMENT EN FECONDATION IN VITRO|Gedeon Richter Plc. |
December 29 2017 | -- |
| NCT03118297 | Completed | Contraception|Bleeding |
Washington University School of Medicine |
May 1 2017 | Phase 3 |
| NCT03156127 | Withdrawn | Uterine Myoma |
Boryung Pharmaceutical Co. Ltd |
May 19 2017 | Phase 1 |
| NCT02859337 | Completed | Obesity|Contraception |
Oregon Health and Science University|National Institutes of Health (NIH) |
May 30 2017 | Phase 4 |
| NCT02408770 | Completed | Breast Cancer |
Manchester University NHS Foundation Trust|University of Manchester |
January 22 2016 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































