research use only
Lersivirine (UK-453061) Reverse Transcriptase inhibitor
Cat.No.S8055
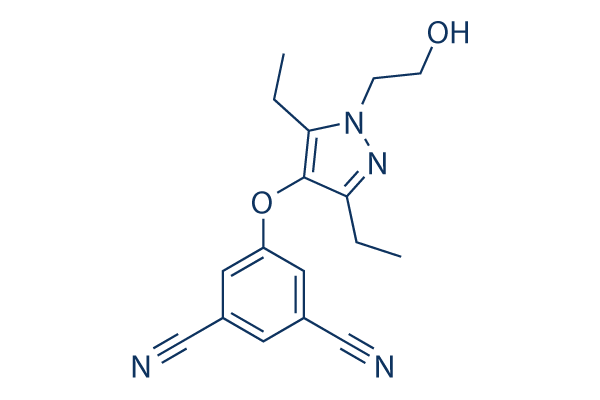
Chemical Structure
Molecular Weight: 310.35
Quality Control
| Related Targets | Integrase Bacterial Antibiotics Anti-infection Fungal Antiviral COVID-19 Parasite HIV HCV Protease |
|---|---|
| Other Reverse Transcriptase Inhibitors | Dapivirine (TMC120) Fangchinoline 3'-Fluoro-3'-deoxythymidine (Alovudine) Salicylanilide Bifendate Ulonivirine 4-Chloro-2-(trifluoroacetyl)aniline hydrochloride |
Solubility
|
In vitro |
DMSO
: 62 mg/mL
(199.77 mM)
Ethanol : 62 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 310.35 | Formula | C17H18N4O2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 473921-12-9 | -- | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | CCC1=C(C(=NN1CCO)CC)OC2=CC(=CC(=C2)C#N)C#N | ||
Mechanism of Action
| Targets/IC50/Ki |
NNRT
|
|---|---|
| In vitro |
Lersivirine (UK-453061) binds to HIV-1 wt reverse transcriptase (RT) with Kd of 624 nM. It is a very weak inhibitor of human DNA polymerase beta, with an extrapolated geometric mean IC50 of approximately 20 mM resulting in a predicted selectivity index of 166,000. This compound is able to inhibit HIV-1 virus replication in MT-2 cells infected with wt NL4-3, with an EC50 ranging from 5 nM to 35 nM against an MOI ranging from 0.005 to 0.5. It retains activity against 80% of viruses with genotypes containing K103N as a single NNRTI mutation (versus 7% for efavirenz), 57% of viruses with genotypes containing Y181C as a single NNRTI mutation (43% for efavirenz), and 46% of viruses with genotypes containing G190A as a single NNRTI mutation (0% for efavirenz). Lersivirine inhibits the replication of strain Ba-L in PBL, with a geometric mean EC50 equal to 3.38 nM (95% CI, 2.26 to 5.05 nM) and an EC90 equal to 9.87 nM (95% CI, 6.63 to 14.7 nM), with no cytotoxicity observed up to 50 μM. Combinations of it with drugs of the NRTI class (abacavir, didanosine, emtricitabine, lamivudine, tenofovir, and zidovudine) results in synergistic interactions. |
| Kinase Assay |
RT enzyme assays
|
|
The activities of lersivirine (UK-453061) and efavirenz against wt RT (BH10 strain) are determined using a primer extension assay incorporating a DNA/RNA primer/template. The 5’biotinylated primer DNA is a 16mer oligo(dT), which is annealed to a poly(rA) template of approximately 300 bases in length. Incorporation of [3H]TTP(dTTP) by reverse transcription results in extension of the primer. During the reaction the primer/template is bound to a streptavidin-coated flashplate (NEN). The incorporated tritiated nucleotides can then stimulate the scintillant to produce a signal that is measured using a scintillation counter. Compounds that inhibit the RNA-dependent DNA polymerase activity of RT produce a reduced signal.
|
|
| In vivo |
In mice, Lersivirine (UK-453061) is not teratogenic. |
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT01220232 | Completed | Healthy Volunteers |
Pfizer|ViiV Healthcare |
November 2010 | Phase 1 |
| NCT01230385 | Completed | Healthy Volunteers |
Pfizer|ViiV Healthcare |
October 2010 | Phase 1 |
| NCT00925535 | Completed | Healthy Volunteers |
Pfizer |
May 2010 | Phase 1 |
| NCT01050751 | Completed | Healthy Volunteers |
Pfizer |
February 2010 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































