research use only
MK-8776 (SCH 900776) Chk inhibitor
Cat.No.S2735
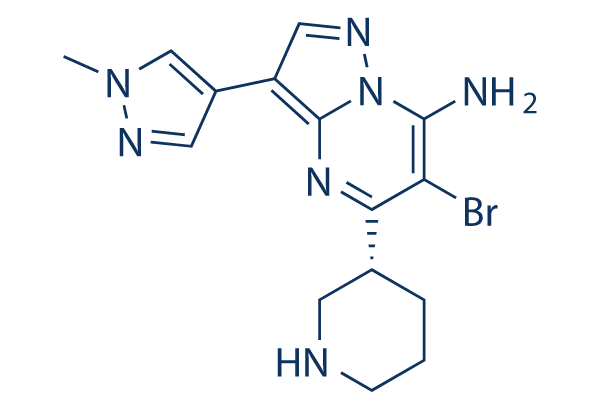
Chemical Structure
Molecular Weight: 376.25
Quality Control
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| SKOV3 | Growth Inhibition Assay | 0.3 µM | 8 d | sensitizes the cell lines to | 23548269 | |
| BxPC-3 | Growth Inhibition Assay | 10-1000 nM | 24-48h | enhances the chemosensitization to | 23804422 | |
| MiaPaCa-2 | Growth Inhibition Assay | 10-1000 nM | 24-48h | enhances the chemosensitization to | 23804422 | |
| AsPC-1 | Growth Inhibition Assay | 10-1000 nM | 24-48h | enhances the chemosensitization to | 23804422 | |
| H1299 | Growth Inhibition Assay | 500 nM | 24 h | DMSO | enhances the chemosensitization to PMX | 24113549 |
| H1993 | Growth Inhibition Assay | 500 nM | 24 h | DMSO | enhances the chemosensitization to PMX | 24113549 |
| H1437 | Growth Inhibition Assay | 500 nM | 24 h | DMSO | enhances the chemosensitization to PMX | 24113549 |
| H23 | Growth Inhibition Assay | 500 nM | 24 h | DMSO | enhances the chemosensitization to PMX | 24113549 |
| AsPC-1 | Growth Inhibition Assay | 200/2000 nM | 24 h | decreases the IC50 of | 24359526 | |
| TK10 | Growth Inhibition Assay | 200/2000 nM | 24 h | decreases the IC50 of | 24359526 | |
| A498 | Growth Inhibition Assay | 200/2000 nM | 24 h | decreases the IC50 of | 24359526 | |
| U20S | Growth Inhibition Assay | 200/2000 nM | 24 h | decreases the IC50 of | 24359526 | |
| SNB19 | Growth Inhibition Assay | 200/2000 nM | 24 h | decreases the IC50 of | 24359526 | |
| MDA-MB-435 | Growth Inhibition Assay | 200/2000 nM | 24 h | decreases the IC50 of | 24359526 | |
| U87 | Growth Inhibition Assay | 200/2000 nM | 24 h | decreases the IC50 of | 24359526 | |
| HCC2998 | Growth Inhibition Assay | 200/2000 nM | 24 h | decreases the IC50 of | 24359526 | |
| MDA-MB-231 | Growth Inhibition Assay | 200/2000 nM | 24 h | decreases the IC50 of | 24359526 | |
| MiaPaCa-2 | Growth Inhibition Assay | 200/2000 nM | 24 h | decreases the IC50 of | 24359526 | |
| MCF10A | Growth Inhibition Assay | 200/2000 nM | 24 h | decreases the IC50 of | 24359526 | |
| HCT116 | Growth Inhibition Assay | 200/2000 nM | 24 h | decreases the IC50 of | 24359526 | |
| IGROV-1 | Growth Inhibition Assay | 200/2000 nM | 24 h | decreases the IC50 of | 24359526 | |
| SW620 | Growth Inhibition Assay | 200/2000 nM | 24 h | decreases the IC50 of | 24359526 | |
| HCT115 | Growth Inhibition Assay | 200/2000 nM | 24 h | decreases the IC50 of | 24359526 | |
| U251 | Growth Inhibition Assay | 200/2000 nM | 24 h | decreases the IC50 of | 24359526 | |
| OVCAR-8 | Growth Inhibition Assay | 0.3 µM | 8 d | sensitizes the cell lines to | 23548269 | |
| MV-4-11 | Apoptosis Assay | 100-700 nM | 48 h | induces apoptosis dose dependently | 23536721 | |
| U937 | Apoptosis Assay | 100-700 nM | 48 h | induces apoptosis dose dependently | 23536721 | |
| MOLM-13 | Apoptosis Assay | 100-700 nM | 48 h | induces apoptosis dose dependently | 23536721 | |
| A2058 | Cell Viability Assay | 37.5-300 nM | 72 h | DMSO | reduces the MK-1775 EC50 by 5-fold to an average of 45 nM | 23148684 |
| H2009 | Cell Viability Assay | 500 nM | 72 h | DMSO | results in G1/S-phase accumulation combined with MK-1775 | 23148684 |
| Su.86.86 | Cell Viability Assay | 500 nM | 72 h | DMSO | results in G1/S-phase accumulation combined with MK-1775 | 23148684 |
| HRE | Cell Viability Assay | 500 nM | 72 h | DMSO | results in G1/S-phase accumulation combined with MK-1775 | 23148684 |
| HMEC | Cell Viability Assay | 500 nM | 72 h | DMSO | results in G1/S-phase accumulation combined with MK-1775 | 23148684 |
| U2OS | Function Assay | 2 µM | 0-24 h | induces phosphorylation of Chk1 at serine 345 at both concentrations as early as 2 h after administration | 22937147 | |
| U2OS | Growth Inhibition Assay | 0-10 µM | 24/48 h | inhibits cell growth dose dependently | 22937147 | |
| U937 | Function Assay | 100-500 nM | 4 h | decreases the cytarabine-induced Chk1 autophosphorylation at Ser296 and prevents Cdc25A downregulation | 22869869 | |
| U937 | Function Assay | 100 nM | 4 h | reverses the cytarabine-induced inhibition of 3H-thymidine incorporation into DNA | 22869869 | |
| U937 | Function Assay | 100-500 nM | 4 h | induces increased phosphorylation of H2AX | 22869869 | |
| HL-60 | Apoptosis Assay | 30/100/300 nM | 24 h | DMSO | enhances cytarabine-induced apoptosis | 22869869 |
| ML-1 | Apoptosis Assay | 25/50/100 nM | 24 h | DMSO | enhances cytarabine-induced apoptosis | 22869869 |
| HCT116 | Function Assay | 1 µM | 24 h | abrogates of cell cycle arrest | 22510560 | |
| U2OS | Function Assay | 1 µM | 24 h | abrogates of cell cycle arrest | 22510560 | |
| Sf9 | Function assay | Inhibition of recombinant CDK2/Cyclin A expressed in insect Sf9 cells assessed as inhibition of [33P]-ATP incorporation into biotinylated histone H1 after 1 hr by liquid scintillation counting, IC50 = 0.16 μM. | 21094607 | |||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 75 mg/mL
(199.33 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 376.25 | Formula | C15H18BrN7 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 891494-63-6 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | CN1C=C(C=N1)C2=C3N=C(C(=C(N3N=C2)N)Br)C4CCCNC4 | ||
Mechanism of Action
| Targets/IC50/Ki |
Chk1
(Cell-free assay) 3 nM
CDK2
(Cell-free assay) 0.16 μM
|
|---|---|
| In vitro |
MK-8776 (SCH 900776) is a less potent inhibitor of Chk2 and CDK2 with IC50 of 1.5 μM and 0.16 μM, respectively. It shows no significant inhibition of cytochrome P450 human liver microsomal isoforms 1A2, 2C9, 2C19, 2D6, and 3A4. In combination with an antimetabolite, this compound induces accumulation of γ-H2AX within 2 hours, indicative of replication fork collapse and double stranded DNA breaks. Additionally, it suppresses accumulation of the Chk1 pS296 autophosphorylation in a dose-dependent manner. Exposure of proliferating WS1 cells to SCH 900776 is associated with rapid, dose-dependent accumulation of Chk1 pS345, indicating that cycling populations of normal cells induce Chk1 pS345 following exposure to it as part of a futile cycle, perhaps driven by AT-family kinases and DNA-PK. |
| Kinase Assay |
Chk1 SPA assay
|
|
An in vitro assay utilizing recombinant His-Chk1 expressed in the baculovirus expression system as an enzyme source and biotinylated peptide based upon CDC25C as substrate. His-Chk1 is diluted to 32 nM in kinase buffer containing 50 mM Tris pH 8.0, 10 mM MgCl2, and 1 mM DTT. CDC25C (CDC25 Ser216 C-term biotinylated peptide) peptide is diluted to 1.93 μM in kinase buffer. For each kinase reaction, 20 μL of 32 nM Chk1 enzyme solution and 20 μL of 1.926 μM CDC25C are mixed and combined with 10 μL of MK-8776 (SCH 900776) diluted in 10% DMSO, making final reaction concentrations of 6.2 nM Chk1, 385 nM CDC25C and 1% DMSO after addition of start solution. The reaction is started by addition of 50 μL of start solution consisting of 2 μM ATP and 0.2 μCi of 33P-ATP, making a final reaction concentration of 1 μM ATP, with 0.2 μCi of 33P-ATP per reaction. Kinase reactions run for 2 hours at room temperature and are stopped by the addition of 100 μL of stop solution consisting of 2 M NaCl, 1% H3PO4, and 5 mg/mL Streptavidin-coated SPA beads. SPA beads are captured using a 96-well GF/B filter plate and a Filtermate universal harvester. Beads are washed twice with 2 M NaCl and twice with 2 M NaCl with 1% phosphoric acid. Signal is then assayed using a TopCount 96-well liquid scintillation counter. Dose-response curves are generated from duplicate 8 point serial dilutions of this compound. IC50 values are derived by nonlinear regression analysis.
|
|
| In vivo |
Dose escalation of MK-8776 (SCH 900776) (16 mg/kg and 32 mg/kg) induces incremental improvements in tumor response. Importantly, doses of this compound associate with robust biomarker activation and improved tumor response are not associated with enhanced toxicity on hematological parameters in BALB/c mice. |
References |
|
Applications
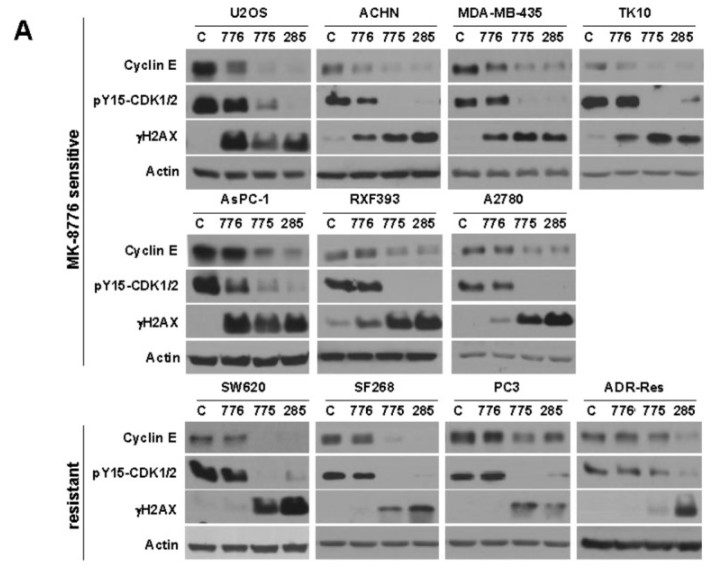
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | Cyclin E / pY15-CDK / γH2AX p-chk1(ser345) / CDC25A |
|
26595527 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT00779584 | Completed | Hodgkin Disease|Lymphoma Non-Hodgkin|Neoplasms |
Merck Sharp & Dohme LLC |
October 17 2008 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
Frequently Asked Questions
Question 1:
I would like to know whether your product S2735 is the optically pure R enantiomer or whether it is a racemic mix.
Answer:
It is the R enantiomer of our S2735.






































