research use only
Montelukast Sodium LTR antagonist
Cat.No.S4211
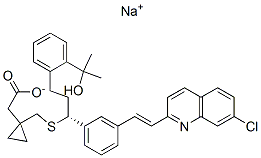
Chemical Structure
Molecular Weight: 608.17
Quality Control
| Related Targets | PD-1/PD-L1 CXCR STING AhR Immunology & Inflammation related CD markers Interleukins Anti-infection Antioxidant COX |
|---|---|
| Other LTR Inhibitors | MK-571 sodium salt LY255283 Etalocib |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| HEK293 | Function assay | 10 mins | Antagonist activity against CysLT1 receptor (unknown origin) expressed in HEK293 cells assessed as inhibition of LTD4-induced calcium mobilization pre-incubated for 10 mins before LTD4 addition by Fluo-4 AM dye based fluorimetric assay, IC50=0.31μM | 26985325 | ||
| HEK293 | Function assay | 10 mins | Antagonist activity against CysLT2 receptor (unknown origin) expressed in HEK293 cells assessed as inhibition of LTD4-induced calcium mobilization pre-incubated for 10 mins before LTD4 addition by Fluo-4 AM dye based fluorimetric assay, IC50=27μM | 26985325 | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(164.42 mM)
Water : 100 mg/mL Ethanol : 100 mg/mL |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 608.17 | Formula | C35H36ClNO3S.Na |
Storage (From the date of receipt) | 3 years -20°C powder |
|---|---|---|---|---|---|
| CAS No. | 151767-02-1 | Download SDF | Storage of Stock Solutions | Solutions are unstable. Prepare fresh or purchase small, pre-packaged sizes. Repackage upon receipt. | |
| Synonyms | MK0476 | Smiles | CC(C)(C1=CC=CC=C1CCC(C2=CC=CC(=C2)C=CC3=NC4=C(C=CC(=C4)Cl)C=C3)SCC5(CC5)CC(=O)[O-])O.[Na+] | ||
Mechanism of Action
| Targets/IC50/Ki |
CysLT1
|
|---|---|
| In vitro |
Montelukast is a leukotriene receptor antagonist (LTRA) used for the maintenance treatment of asthma and to relieve symptoms of seasonal allergies. Montelukast blocks the action of leukotriene D4 on the cysteinyl leukotriene receptor CysLT1 in the lungs and bronchial tubes by binding to it, preventing airway edema, smooth muscle contraction, and enhanced secretion of thick, viscous mucus. This reduces the bronchoconstriction otherwise caused by the leukotriene, and results in less inflammation. |
| In vivo |
Montelukast (6 mg/kg, once per day for 20 d) significantly suppresses the increased eosinophils in BAL fluid and lung tissue, and increases IL-5 level in BAL fluid in OVA challenged mice. OVA challenge increases CysLT1 but decreases CysLT2 receptor mRNA expression. Montelukast inhibits the increased CysLT1 but not the reduces CysLT2 expression after OVA challenge. |
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06340984 | Not yet recruiting | Acne Vulgaris |
South Valley University |
June 2024 | Phase 3 |
| NCT04351698 | Recruiting | Anemia Sickle Cell|Sleep-Disordered Breathing |
Great Ormond Street Hospital for Children NHS Foundation Trust|Guy''s and St Thomas'' NHS Foundation Trust|University College London Hospitals|North Middlesex University Hospital|King''s College Hospital NHS Trust|The Whittington Hospital NHS Trust |
October 16 2023 | Phase 2|Phase 3 |
| NCT05651750 | Unknown status | To Evaluate PSQ as Clinical Tool in the Decision Between Medical and Surgical Treatment for Adenotonsillar Hypertrophy|To Determine Clinical Response to Montelukast or Nasal Steroids Based on PSQ Results |
Assaf-Harofeh Medical Center |
November 15 2022 | Phase 4 |
| NCT05064631 | Recruiting | Respiratory Tract Infections|Pediatric Respiratory Diseases|Wheezing |
Queen Mary University of London|Queensland University of Technology |
January 12 2022 | Phase 2 |
| NCT05710081 | Recruiting | Wheezing|Bronchiolitis|Asthma in Children |
Barts & The London NHS Trust|University of Aberdeen|University of Edinburgh|King''s College London|University of Southampton|Menzies School of Health Research |
November 30 2021 | Phase 3 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































