research use only
Atazanavir HIV Protease inhibitor
Cat.No.S4662
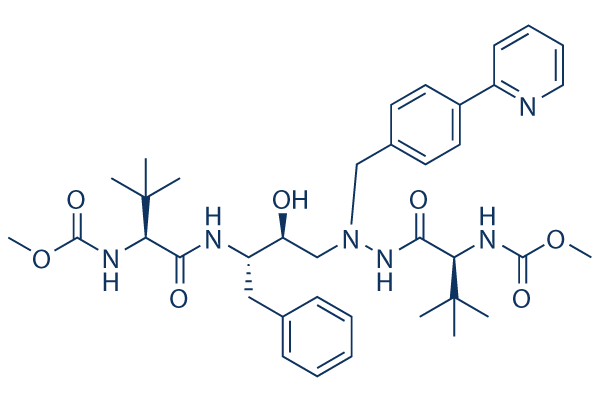
Chemical Structure
Molecular Weight: 704.86
Quality Control
| Related Targets | HDAC Caspase Proteasome Secretase MMP HCV Protease Cysteine Protease Tyrosinase DPP Serine Protease |
|---|---|
| Other HIV Protease Inhibitors | Pepstatin A Limonin Temsavir (BMS-626529) Rosamultin Bevirimat Dextran sulfate sodium (DSS) Mericitabine NBD-556 GS-6207 (Lenacapavir) Azvudine |
Solubility
|
In vitro |
DMSO
: 60 mg/mL
(85.12 mM)
Ethanol : 24 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 704.86 | Formula | C38H52N6O7 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 198904-31-3 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | Latazanavir, Zrivada, Reyataz, BMS-232632 | Smiles | CC(C)(C)C(C(=O)NC(CC1=CC=CC=C1)C(CN(CC2=CC=C(C=C2)C3=CC=CC=N3)NC(=O)C(C(C)(C)C)NC(=O)OC)O)NC(=O)OC | ||
Mechanism of Action
| Targets/IC50/Ki |
HIV protease
|
|---|---|
| In vitro |
Atazanavir has potent in vitro activity with 50 and 90% effective concentrations(EC50) of 2-5 nM and 9-15 nM respectively against wild type virus. This compound is able to potently induce endoplasmic reticulum (ER) stress response in malignant glioma cells, as indicated by elevated levels of GRP78 and CHOP, and activation of caspase-4, which leads to cell death.
|
| In vivo |
Atazanavir has excellent oral bioavailability in the range of 60-70%.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT04121195 | Active not recruiting | HIV/AIDS|Tuberculosis |
University of Liverpool|European and Developing Countries Clinical Trials Partnership (Funder)|Joint Clinical Research Centre Kampala Uganda|University of Cape Town Cape Town South Africa|Infectious Diseases Institute Makerere University College of Health Sciences Kampala Uganda|University of Turin Turin Italy |
October 30 2020 | Phase 2|Phase 3 |
| NCT02697851 | Terminated | HIV |
St Stephens Aids Trust|Bristol-Myers Squibb |
July 2016 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































