research use only
Hydroxyurea Ribonucleotide Reductase Inhibitor
Cat.No.S1896
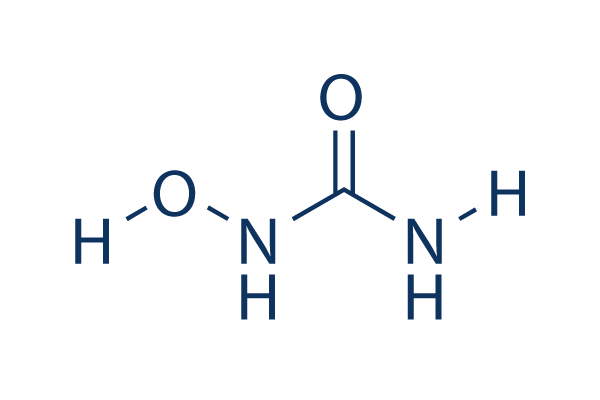
Chemical Structure
Molecular Weight: 76.05
Quality Control
| Related Targets | HDAC PARP ATM/ATR DNA-PK WRN Topoisomerase PPAR Sirtuin Casein Kinase eIF |
|---|---|
| Other DNA/RNA Synthesis Inhibitors | B02 CX-5461 (Pidnarulex) SCR7 EED226 Favipiravir (T-705) RK-33 Carmofur Triapine (3-AP) BMH-21 YK-4-279 |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| L1210 Leukemia cells | Growth inhibition assay | Concentration required for 50% inhibition of cell growth (L1210 Leukemia), IC50=21.3796 μM | 2991520 | |||
| P388 leukemic cell | Proliferation assay | 24-48 h | Antiproliferative activity of compound expressed as concentration that inhibits 50% of growth of P388 leukemic cell suspension from 24 to 48 hr after compound addition, IC50=32 μM | 2709372 | ||
| L1210 | Function assay | 48 h | Activity against cultured L1210 leukemic cells was determined in vitro, after 48 h of incubation. Compound dose that causes 16 % inhibition was reported, ID16 = 1.99 μM. | 6319702 | ||
| U373-MAGI | Function assay | 500 uM | 2 hrs | Potentiation of 5-Aza-C-induced antiviral activity against VSV-G pseudotyped HIV-1 NL4-3 infected in human U373-MAGI cells assessed as 5-Aza-C EC50 at 500 uM preincubated for 2 hrs followed by 5-Aza-C addition for 2 hrs and subsequent viral infection meas, EC50 = 25.8 μM. | 27117260 | |
| Burkitt's lymphoma cells | Function assay | Inhibition of [14C]-cytidine incorporation into DNA in Burkitt's lymphoma cells, IC50 = 37.15 μM. | 11405653 | |||
| U373-MAGI | Antiviral assay | 500 uM | Antiviral activity against VSV-G pseudotyped HIV-1 NL4-3 infected in human U373-MAGI cells assessed as reduction in viral infectivity at 500 uM incubated for 4 hrs prior to viral infection measured at 72 hrs post infection by flow cytometric analysis | 27117260 | ||
| U373-MAGI | Function assay | 0.5 mM | 2 hrs | Increase in 5-aza-dCTP/dCTP ratio in human U373-MAGI cells at 0.5 mM preincubated for 2 hrs followed by 5-aza-C addition measured after 4 hrs by LC-MS/MS analysis relative to 5-aza-C | 27117260 | |
| U373-MAGI | Function assay | 2 mM | 6 hrs | Reduction in dATP level in human U373-MAGI cells at 2 mM after 6 hrs by LC-MS/MS analysis | 27117260 | |
| U373-MAGI | Function assay | 0.5 mM | 2 hrs | Reduction in dCTP level in human U373-MAGI cells at 0.5 mM preincubated for 2 hrs followed by 5-aza-C addition measured after 4 hrs by LC-MS/MS analysis | 27117260 | |
| U373-MAGI | Function assay | 2 mM | 2 hrs | Reduction in dCTP level in human U373-MAGI cells at 2 mM preincubated for 2 hrs followed by 5-aza-C addition measured after 4 hrs by LC-MS/MS analysis relative to 5-aza-C | 27117260 | |
| U373-MAGI | Function assay | 0.5 mM | 2 hrs | Increase in 5-aza-dCTP/dCTP ratio in human U373-MAGI cells at 0.5 mM preincubated for 2 hrs followed by 5-aza-dC addition measured after 4 hrs by LC-MS/MS analysis relative to 5-aza-dC | 27117260 | |
| U373-MAGI | Function assay | 2 mM | 2 hrs | Increase in 5-aza-dCTP/dCTP ratio in human U373-MAGI cells at 2 mM preincubated for 2 hrs followed by 5-aza-dC addition measured after 4 hrs by LC-MS/MS analysis relative to 5-aza-dC | 27117260 | |
| U373-MAGI | Function assay | 0.5 mM | 2 hrs | Reduction in dCTP level in human U373-MAGI cells at 0.5 mM preincubated for 2 hrs followed by 5-aza-dC addition measured after 4 hrs by LC-MS/MS analysis relative to 5-aza-dC | 27117260 | |
| U373-MAGI | Function assay | 2 mM | 2 hrs | Reduction in dCTP level in human U373-MAGI cells at 2 mM preincubated for 2 hrs followed by 5-aza-dC addition measured after 4 hrs by LC-MS/MS analysis relative to 5-aza-dC | 27117260 | |
| TC32 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for TC32 cells | 29435139 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | |||
| Saos-2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Saos-2 cells | 29435139 | |||
| BT-37 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-37 cells | 29435139 | |||
| RD | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for RD cells | 29435139 | |||
| BT-12 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-12 cells | 29435139 | |||
| MG 63 (6-TG R) | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for MG 63 (6-TG R) cells | 29435139 | |||
| OHS-50 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for OHS-50 cells | 29435139 | |||
| Rh41 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh41 cells | 29435139 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for A673 cells) | 29435139 | |||
| U-2 OS | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for U-2 OS cells | 29435139 | |||
| SJ-GBM2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SJ-GBM2 cells | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 29435139 | |||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 29435139 | |||
| TC32 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for TC32 cells | 29435139 | |||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 15 mg/mL
(197.23 mM)
Water : 15 mg/mL Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 76.05 | Formula | CH4N2O2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 127-07-1 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | NCI-C04831, Hydroxycarbamide,NSC-32065 | Smiles | C(=O)(N)NO | ||
Mechanism of Action
| Targets/IC50/Ki |
ribonucleoside diphosphate reductase
|
|---|---|
| In vitro |
hydroxyurea can inhibit HIV-1 replication. In vitro experiments have shown that the 90% inhibitory concentration (IC90) of this compound for laboratory strains of HIV-1 in activated PBMC is 0.4 mM. It was also found to be synergistic with the nucleoside reverse transcriptase inhibitor didanosine and to inhibit HIV-1 replication in activated PBMC; this inhibition may be due to a reduction in deoxynucleoside triphosphate pool sizes. This chemical has been shown to sensitize didanosine-resistant mutants. It has demonstrated activity in the treatment of sickle cell anemia by increasing the production of fetal hemoglobin, which reduces hemolysis in patients with this disease. This agent exerts its cytostatic effect through inhibition of ribonucleotide reductase—the rate-limiting enzyme responsible for the conversion of ribonucleotides to deoxyribonucleotides, which are essential for DNA synthesis. As a result, cellular division is arrested in the S phase.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06171217 | Recruiting | Sickle Cell Disease|Children |
Children''s Hospital Medical Center Cincinnati|National Heart Lung and Blood Institute (NHLBI) |
October 27 2023 | Phase 2 |
| NCT05909657 | Recruiting | Sickle Cell Disease |
The University of The West Indies |
July 1 2023 | -- |
| NCT05548062 | Recruiting | Polycythemia Vera |
Novartis Pharmaceuticals|Novartis |
March 2 2023 | -- |
| NCT05662098 | Recruiting | Sickle Cell Disease |
Children''s Hospital Medical Center Cincinnati|Jinja Regional Referral Hospital (JRRH) Sickle Cell Clinic Jinja Uganda |
June 16 2022 | Early Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































