research use only
Inosine
Cat.No.S2442
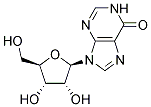
Chemical Structure
Molecular Weight: 268.23
Quality Control
Solubility
|
In vitro |
DMSO
: 53 mg/mL
(197.59 mM)
Water : 47 mg/mL Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 268.23 | Formula | C10H12N4O5 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 58-63-9 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | NSC 20262, INO 495 | Smiles | C1=NC2=C(C(=O)N1)N=CN2C3C(C(C(O3)CO)O)O | ||
Mechanism of Action
| In vitro |
Inosine is a nucleoside that is formed when hypoxanthine is attached to a ribose ring (also known as a ribofuranose) via a β-N9-glycosidic bond. This compound is commonly found in tRNAs and is essential for proper translation of the genetic code in wobble base pairs. Knowledge of its metabolism has led to advances in immunotherapy in recent decades. Its monophosphate is oxidised by the enzyme inosine monophosphate dehydrogenase, yielding xanthosine monophosphate, a key precursor in purine metabolism.
|
|---|---|
| In vivo |
LD50: Mice >6g/kg (i.g.)
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT04476238 | Completed | Healthy Volunteers |
University Hospital Basel Switzerland |
February 28 2022 | Not Applicable |
| NCT01726400 | Completed | Hepatitis C |
Fremantle Hospital and Health Service |
December 2012 | -- |
| NCT01675427 | Completed | Hepatitis C Chronic |
Hoffmann-La Roche |
August 2011 | Phase 4 |
| NCT01639534 | Completed | Identify the Presence of a Marker of Ischemia/Hypoperfusion of the Brain Via Peripheral Blood|Ischemia/Hypoperfusion of Brain |
Virginia Commonwealth University |
March 2011 | -- |
| NCT00978965 | Completed | Renal Transplantation |
Medical University of Vienna|Novartis |
October 2009 | -- |
| NCT00923728 | Withdrawn | Refractory Solid Tumors |
Vertex Pharmaceuticals Incorporated|National Cancer Institute (NCI) |
April 2009 | Not Applicable |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































