research use only
Conivaptan HCl Vasopressin Receptor antagonist
Cat.No.S2116
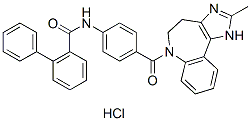
Chemical Structure
Molecular Weight: 535.04
Quality Control
| Related Targets | CXCR Hedgehog/Smoothened PKA Adrenergic Receptor AChR 5-HT Receptor Histamine Receptor Dopamine Receptor Ras KRas |
|---|---|
| Other Vasopressin Receptor Inhibitors | Mozavaptan RO 5028442 (RG7713) Lixivaptan (VPA-985) Mozavaptan (hydrochloride) |
Solubility
|
In vitro |
DMSO
: 107 mg/mL
(199.98 mM)
Ethanol : 7 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 535.04 | Formula | C32H26N4O2.HCl |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 168626-94-6 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | YM 087 | Smiles | CC1=NC2=C(N1)CCN(C3=CC=CC=C32)C(=O)C4=CC=C(C=C4)NC(=O)C5=CC=CC=C5C6=CC=CC=C6.Cl | ||
Mechanism of Action
| Targets/IC50/Ki |
Vasopressin receptor 1
Vasopressin receptor 2
|
|---|---|
| In vivo |
Conivaptan (0.03, 0.1 and 0.3 mg/kg i.v.) dose-dependently increases urine volume and reduces urine osmolality in both myocardial infarction and sham-operated rats. Conivaptan (0.3 mg/kg i.v.) significantly reduces right ventricular systolic pressure, left ventricular end-diastolic pressure, lung/body weight and right atrial pressure in myocardial infarction rats. Conivaptan (0.3 mg/kg i.v.) significantly increases dP/dt(max)/left ventricular pressure in myocardial infarction rats. Conivaptan produces an acute increase in urine volume (UV), a reduction in osmolality (UOsm) and, at the end of the investigation, cirrhotic rats receiving the V(1a)/V(2)-AVP receptor antagonist does not show hyponatremia or hypoosmolality. Conivaptan also normalizes U(Na)V without affecting creatinine clearance and arterial pressure. Conivaptan (0.01 to 0.1 mg/kg i.v.) exerts a dose-dependent diuretic effect in dogs without an increase in the urinary excretion of electrolytes, inhibits the pressor effect of exogenous vasopressin in a dose-dependent manner (0.003 to 0.1 mg/kg i.v.) and, at the highest dose (0.1 mg/kg i.v.), almost completely blocks vasoconstriction caused by exogenous vasopressin. Conivaptan (0.1 mg/kg i.v.) improves cardiac function, as evidenced by significant increases in left ventricular dP/dtmax, cardiac output and stroke volume, and reduces preload and afterload, as evidenced by significant decreases in left ventricular end-diastolic pressure and total peripheral vascular resistance in dogs with congestive heart failure. |
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT03000283 | Completed | Cerebral Hemorrhage|Cerebral Edema|Intracerebral Hemorrhage|Stroke |
Jesse Corry|Allina Health System |
March 22 2017 | Phase 1 |
| NCT01370148 | Completed | Liver Disease |
Cumberland Pharmaceuticals |
April 2011 | Phase 1 |
| NCT00887627 | Completed | Kidney Diseases|Hyponatremia |
Cumberland Pharmaceuticals |
April 2009 | Phase 1 |
| NCT00851227 | Completed | Liver Disease|Hyponatremia |
Cumberland Pharmaceuticals |
February 2009 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































